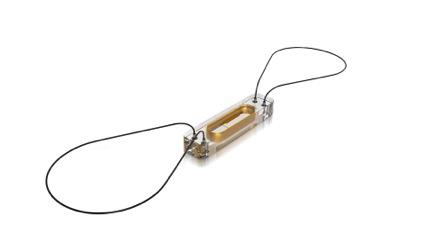
Important new data presented during the Heart Rhythm Society's (HRS) 36th annual Scientific Session supporting improved outcomes and cost-effectiveness of the CardioMEMS HF System for the management of Class III heart failure patients.
Biotronik announced that since the launch of the ProMRI Eluna pacemaker system in late March 2015, 60 percent of pacing devices sold by the company are approved for use during magnetic resonance imaging (MRI) scans. The announcement was made at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions, May 13-16 in Boston.
The Heart Rhythm Society (HRS) has released a first-of-its-kind expert consensus statement on three specific cardiovascular disorders that also involve the autonomic nervous system. The 2015 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Treatment of Postural Tachycardia Syndrome, Inappropriate Sinus Tachycardia, and Vasovagal Syncope was written by an international group of experts and published online in HeartRhythm Journal, the official journal of HRS. The expert consensus statement was presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Janssen Pharmaceuticals Inc. and its development partner Bayer HealthCare announced results from the VENTURE-AF trial. The study explored the potential of once-daily Xarelto (rivaroxaban) as an alternative to vitamin K antagonists (VKA), used to reduce the risk of blood clots, in people with non-valvular atrial fibrillation (NVAF) undergoing catheter ablation, a frequently used interventional procedure to remove abnormal tissue in the heart that is causing the irregular heartbeat. The results were presented at Heart Rhythm 2015, the Heart Rhythm Society's 36th annual scientific sessions, and published in the European Heart Journal.
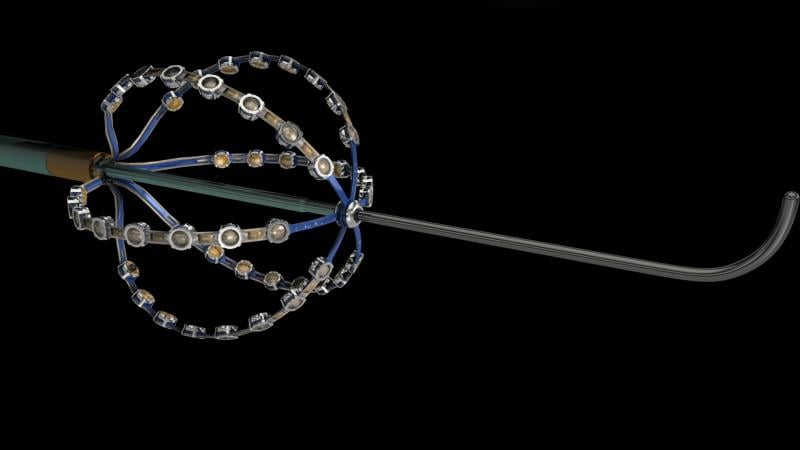
A preliminary study of a new electrophysiology (EP) mapping system using a specialized intracardiac ultrasound basket catheter for electro-mapping showed it is four times higher resolution than standard voltage mapping. Researchers also said the new mapping modality represents the electromapping data on computed tomography (CT) quality anatomy. The data suggest it may open the possibility to map irregular arrhythmias with more precision. The data was presented at Heart Rhythm Society’s 2015 meeting.
Medtronic plc announced that its Tyrx Antibacterial Envelope reduces major cardiac device site infections by 80 percent up to 12 months after implantation. These data were presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions in Boston.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Amaranth Medical announced plans to report clinical results from the company's ongoing trial of its second-generation Fortitude sirolimus-eluting bioresorbable scaffold (BRS) at EuroPCR 2015. Juan F. Granada, M.D., executive director and chief innovation officer of the CRF-Skirball Center for Innovation (SCI), will present these updates.

The first-in-human trial of a new miniaturized leadless pacemaker implanted directly inside the heart found the Medtronic Micra transcatheter pacing system (TPS) safe and effective in patients with a slow heart rhythm. Early performance results from the international Micra Transcatheter Pacing Study were presented at Heart Rhythm 2015, the Heart Rhythm Society’s 36th annual scientific sessions, May 13-16 in Boston.
Clinical trial results showed that full-body magnetic resonance imaging (MRI) scans do not affect the Medtronic Evera MRI SureScan implantable cardioverter defibrillator’s (ICD) ability to detect potentially lethal heart rhythms and deliver life-saving therapy. Data were presented during a late-breaking clinical trial session at Heart Rhythm 2015
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Kalorama Information expects the nearly 25 billion-dollar market for electronic medical records (EMR) to grow well past the period where there are incentives for U.S. healthcare providers, according to a recent report. The healthcare market research publisher said penalties, system upgrades, international sales and continued efficiency improvements will grow the market 7-8 percent each year for the next five years. The market for EMR systems continues to increase as more physicians and hospitals use EMR and acquire EMR systems, and as hospitals and physician groups upgrade existing systems.

Adding the HeartFlow FFRCT Analysis to a standard coronary computed tomography angiogram (cCTA) may change the course of treatment in more than one-third of patients with coronary artery disease. This conclusion was discussed in a study presented at the EuroPCR 2015 conference.
A study presented at Heart Rhythm 2015 found that botulinum toxin (botox) injections into epicardial fat pads during coronary artery bypass graft (CABG) surgery can be beneficial for the patient. The injection not only reduces the incidence of post-operative atrial fibrillation (AF), but also provides substantial AF suppression after one year.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
New data from St. Jude Medical found that patients with cardiac devices who use remote monitoring have significantly fewer hospitalizations and lower overall healthcare costs than patients who do not. The data was presented during a late-breaking clinical trial session during Heart Rhythm 2015, the Heart Rhythm Society’s annual scientific sessions. The findings were a result of a five-year study, one of the largest to date on remote monitoring technologies.
Chest pain sends more than 7 million Americans to the emergency department each year. About half of them are admitted to the hospital for further observation, testing or treatment. Now, emergency medicine physicians at The Ohio State University Wexner Medical Center and Mount Carmel Health System believe that number can be significantly reduced.
A new epicardial pacing lead has been cleared by the U.S. Food and Drug Adminitysration (FDA) as an option for the implant of a pacemaker, defibrillator, or cardiac resynchronization therapy (CRT) device. The lead is indicated when other types of leads cannot be implanted. Examples include patients who have small veins, congenital heart disease, abnormalities of the tricuspid valve or when other leads are already in place, preventing additional leads in the veins.

 May 21, 2015
May 21, 2015











