Biotronik announced a partnership with Maquet Medical Systems USA to distribute Biotronik peripheral vascular devices in the United States.
St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval of the first-to-market MultiPoint Pacing technology featured on the Quadra line of cardiac resynchronization therapy defibrillators (CRT-D) and pacemakers.
February 17, 2016 — Up to 10 percent of people with high blood pressure have resistant hypertension — high blood ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
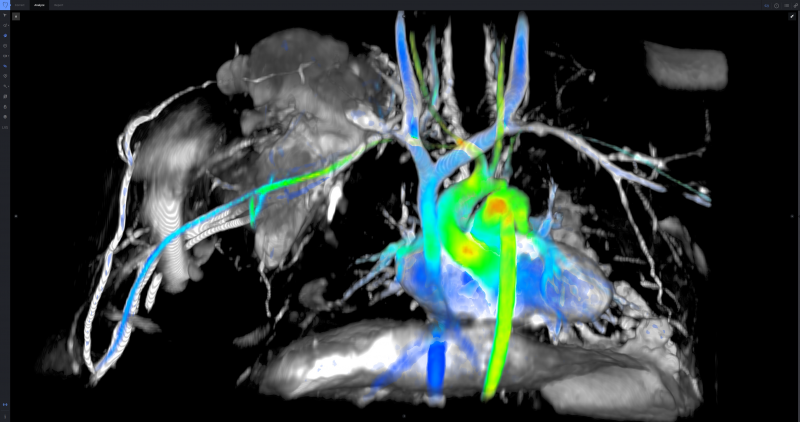
For decades now, magnetic resonance imaging (MRI) has been noted for its excellent soft tissue imaging capability with ...
Apervita Inc. announced the American College of Cardiology (ACC) will add a recognized tool for predicting atherosclerotic cardiovascular disease (ASCVD) to the growing body of analytics being distributed through the Apervita Marketplace.
By genetically reprogramming the most common type of cell in mammalian connective tissue, researchers at the University of Wisconsin-Madison have generated master heart cells — primitive progenitors that form the developing heart.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

(Read a 2018 update on cardiac ultrasound technologies "Recent Advances in Echocardiography Technology") There were some ...
Researchers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) used ultrasound-activated microbubbles to improve preservation of heart muscle and function in a pig heart attack model. Based on this success, the method is now in phase I human clinical trials.
Royal Philips announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the Expression MR400, a new technology that monitors patients undergoing magnetic resonance imaging (MRI).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 10, 2016 — Fred Hoiberg, head coach of the National Basketball Assocation’s (NBA) Chicago Bulls, is launching a ...

After five years of almost constant lobbying efforts and numerous attempts by the U.S. House to push through a repeal of ...
CeloNova BioSciences Inc. announced this week that the first patient has been enrolled in its COBRA REDUCE trial. The COBRA REDUCE trial recently received conditional U.S. Food and Drug Administration (FDA) approval and will study the Cobra PzF nanocoated coronary stent (NCS) system in patients at high risk of bleeding.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
February 10, 2016 — HeartWare International Inc. and Valtech Cardio Ltd. announced the termination of HeartWare's ...
In a finding that could lead to new drugs to treat heart failure, researchers have uncovered the molecular mechanism that regulates how the heart pumps blood.
February 9, 2016 — Cigna has entered into an outcomes-based contract with the pharmaceutical company Novartis for the ...

 February 17, 2016
February 17, 2016