July 27, 2016 — Penumbra Inc. announced U.S. commercial availability of its latest thrombectomy device, the ACE68 ...
Edwards Lifesciences Corp. announced that Health Canada has approved the Edwards Sapien 3 transcatheter heart valve for the treatment of patients living with severe, symptomatic aortic stenosis and at high or greater risk for surgical aortic valve replacement.
July 27, 2016 — A number of states mandate public reporting of mortality outcomes following certain cardiac procedures ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 26, 2016 — A new study has identified a genetic error that weakens the aorta, placing patients with this and ...
On July 20, Massoud Leesar, M.D., of University of Alabama at Birmingham Hospital implanted a patient with Absorb, the world’s first U.S. Food and Drug Administration (FDA)-approved dissolving heart stent, for the first time in Alabama.
July 25, 2016 — The Centers for Medicare and Medicaid Services (CMS) announced that it expects to launch its new Overall ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 26, 2016 — Hematology researchers at The Children’s Hospital of Philadelphia (CHOP) have developed a novel ...
July 26, 2016 — The Centers for Medicare & Medicaid Services (CMS) has proposed the creation of new bundled payment ...

With the recent introduction of several novel oral anticoagulants (NOACs) on the U.S. market that have been billed by ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Stryker Sustainability Solutions (formerly Ascent Healthcare Solutions) is recalling Angiodynamics Soft Vu Omni Flush Angiographic Catheters due to reports of separation of the tip of the catheter from the main body.
UltraSPECT Inc. announced recently that Robert Wood Johnson Physician Enterprise (RWJPE), a multi-specialty, community-based physician group in Central New Jersey, implemented UltraSPECT’s Xpress3.Cardiac solution at four of their sites.
July 22, 2016 — The Centers for Medicare & Medicaid Services (CMS) recently announced 516 awardees in 47 states, Puerto ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
CardioKinetix Inc. announced this week that 500 patients have received the company’s Parachute Ventricular Partitioning Device for heart failure. Patients have been treated in more than 15 countries, including patients in key international markets where the device is commercially available, and patients enrolled in PARACHUTE IV, the company’s U.S. pivotal trial under investigational device exemption (IDE).
People who develop heart failure after their first heart attack have a greater risk of developing cancer when compared to first-time heart attack survivors without heart failure, according to a recent study in the Journal of the American College of Cardiology.
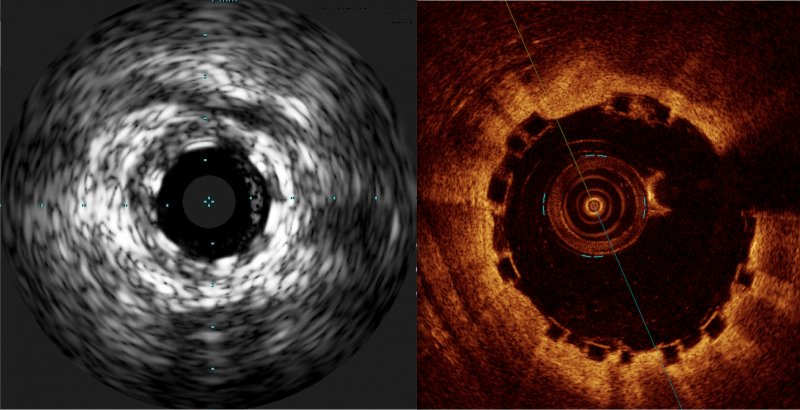
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of ...


 July 27, 2016
July 27, 2016