October 5, 2017 — To provide lifesaving surgical care for sick children in one of the most underserved countries, 13 ...
BTG plc announced it has acquired Roxwood Medical, provider of advanced cardiovascular specialty catheters used in the treatment of patients with severe coronary and peripheral artery disease.

Consolidation of data in one location to improve efficiency and enable data analytics, as well as smooth integration ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
TVA Medical Inc. announced that its everlinQ 4 endoAVF System has received CE Mark in the European Union. The technology uses a 4 French catheter system with enhanced visual indicators to create hemodialysis access using an endovascular technique without open surgery.
October 4, 2017 — MyoKardia Inc. recently provided a clinical update on its MYK-491 program for dilated cardiomyopathy ...
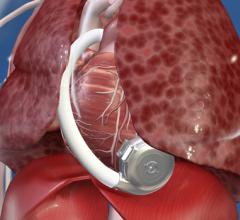
October 4, 2017 — Medtronic received U.S. Food and Drug Administration (FDA) approval for its HeartWare HVAD System as a ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Despite expectations to the contrary, a new survey from cardiovascular healthcare consulting firm MedAxiom finds that overall, cardiology compensation continues to increase. The company’s fifth annual Cardiovascular Provider Compensation and Production Survey also revealed that one in five cardiologists is over the age of 61, and that overall compensation for private cardiologists is increasing faster than that of their integrated counterparts; the differential in compensation is at its lowest point in five years.
October 3, 2017 – Medical Metrics, an experienced core laboratory for multi-center clinical trials, has implemented the ...
October 2, 2017 — Cardiovascular healthcare membership organization and performance community MedAxiom announced its ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 2, 2017 — Reflow Medical Inc. announced that the company has received 510(k) clearance from the U.S. Food and ...
Medtronic plc recently announced a new post-market clinical study to evaluate its CoreValve Evolut Pro valve in everyday clinical practice. Studying patients with severe symptomatic aortic stenosis at an intermediate, high or extreme risk for open-heart surgery, the FORWARD PRO Clinical Study will evaluate longer-term performance (out to five years) of the next-generation self-expanding transcatheter aortic valve implantation (TAVI) system, which was recently approved for commercial use in Europe and United States.
October 2, 2017 — Boston Scientific announced a definitive agreement to acquire Apama Medical Inc., a privately-held ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

October 2, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
September 29, 2017 — TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the premarket ...
Avinger Inc. recently announced Conformité Européenne (CE) Marking approval for treating in-stent restenosis with the Pantheris Lumivascular atherectomy system.

 October 05, 2017
October 05, 2017