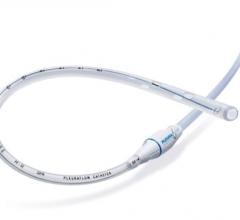
September 11, 2017 — Veniti Inc. announced that Boston Scientific will distribute the Vici Venous Stent under a limited ...
September 11, 2017 — Researchers have led a retrospective single-center study examining simple hemodynamic parameters ...
A cardiovascular service line manager reader of DAIC recently e-mailed me and asked if I had a list of the top ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 8, 2017 — ClearFlow Inc. announced recently that positive clinical trial results were presented at the ...

September 8, 2017 — Abbott Vascular has announced it will end commercial sales of its Absorb bioresorbable vascular ...
September 7, 2017 — Sapheneia and Scannerside received U.S. Food and Drug Administration (FDA) 510(k) clearance to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) announced it would hold a meeting of the Medical Imaging Drugs Advisory Committee (MIDAC) on Sept. 8 to discuss regulatory approaches for use of gadolinium-based contrast agents (GBCAs).

September 7, 2017 — Closure of the left atrial appendage (LAA) during heart surgery protects the brain, according to ...

September 7, 2017 — Oxygen therapy does not improve survival in patients with heart attack symptoms, according to late ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

September 7, 2017 — Researchers at the European Society of Cardiology (ESC) Congress called for a reconsideration of ...
In July, The Heart Institute at Florida Medical Center became the first hospital in the state of Florida to offer the U.S. Food and Drug Administration (FDA)-cleared high sensitive Elecsys Troponin T Gen 5 blood test (Troponin T Gen 5 or TnT Gen 5) to aid in the diagnosis of heart attacks. The new nine-minute test offers faster answers clinicians can use when diagnosing heart attack.
Protembis GmbH announced the first clinical applications of its ProtEmbo Cerebral Protection System to complement a transcatheter aortic valve replacement (TAVR) procedure. The ProtEmbo System is an intra-aortic filter device that deflects embolic material arising during TAVR away from the brain.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The U.S. Food and Drug Administration (FDA) has cleared TrueFusion, a new cardiovascular application from Siemens Healthineers that integrates ultrasound and angiography to guide cardiac teams when administering treatment for structural heart disease. Available on the new Release 5.0 of the Acuson SC2000 cardiovascular ultrasound system, TrueFusion is designed to maximize not only interventional cardiology procedures, but also routine diagnosis and follow-up of patients with structural heart disease.
September 6, 2017 — Physicians identified a majority of patients with advanced heart failure as at high risk for ...
A new National Institutes of Health-funded hypertension trial will examine the possibility of using an emergency department setting to better identify people from central cities with limited access to other venues for medical care.


 September 11, 2017
September 11, 2017