June 29, 2020 — Boston Scientific received U.S. Food and Drug Administration (FDA) 510(k) clearance for the LUX-Dx ...
June 29, 2020 — A type of smart magnetic resonance imaging (MRI) scan used in people with heart disease could help ...
June 26, 2020 — Abbott announced new data from the company's LightLab Initiative that showed optical coherence ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 26, 2020 – New data from the Global SYMPLICITY Registry (GSR) showed that renal denervation (RDN) with the ...
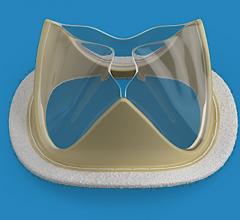
June 23, 2020 – Heart valve start-up Foldax is looking to reinventing several aspects of the prosthetic heart valve ...
June 23, 2020 — Philips announced the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) for ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 23, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Program status for the ...
Prior to January 2020 when clinicians read about the history of the 1918 flu, and epidemiologists predicted we were ...
June 19, 2020 — SMT (Sahajanand Medical Technologies Pvt. Ltd) said it acquired of the structural heart medical device ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
June 19, 2020 — iVascular SLU announced the global launch of Essential Pro, a novel coronary artery drug-coated balloon ...
June 17, 2020 — MedAlliance announced its second CE mark approval for its Selution SLR 0.014 percutaneous transluminal ...
June 16, 2020 — The 36-month results from Veryan Medical’s MIMICS-2 study for the BioMimics 3D femoropopliteal stent ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
June 16, 2020 – The Montreal Heart Institute (MHI) and Thermedical, a developer of thermal-ablation systems to treat ...

June 15, 2020 — The U.S. Food and Drug Administration (FDA) today revoked the emergency use authorization (EUA) that ...


 June 29, 2020
June 29, 2020