Biotronik announced that a unique physiologic sensor is now available in the latest family of cardiac resynchronization therapy defibrillator (CRT-D) devices. Closed Loop Stimulation (CLS) is the only cardiac sensor capable of appropriately adapting heart rate in response to physiologic demands independent of body movements or respiratory rate.
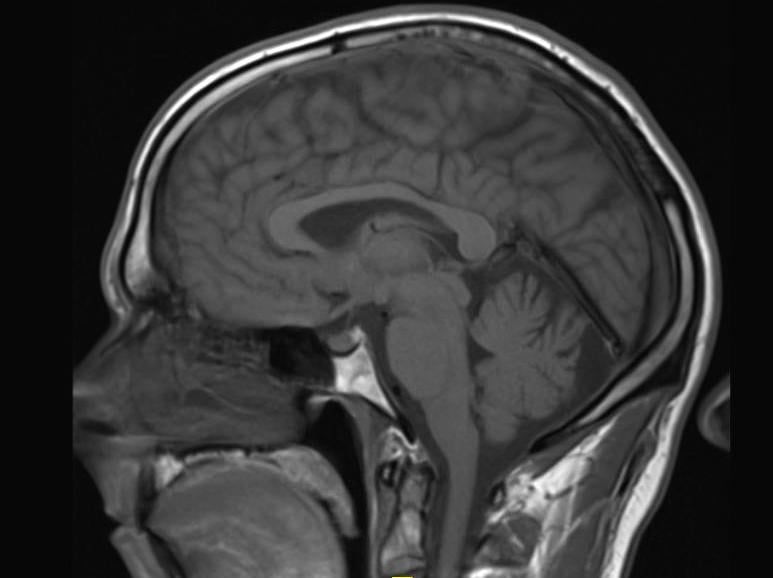
New research, published in the journal Radiology, suggests some types of a popular contrast agent used in magnetic resonance imaging (MRI) exams may remain in the brain for years, but that the long-term effects are unknown.
Carestream showcased the advanced interoperability of its Clinical Collaboration Platform (CCP) at the IHE-Europe (Integrating the Healthcare Enterprise) Connectathon held April 20-24 in Luxembourg. Carestream announced that the CCP product suite successfully passed all integration tests at the Connectathon including all profiles for its universal viewer, vendor-neutral archive (VNA) and image capture solutions. Carestream is also among the first VNA suppliers to implement HL7's new Fast Healthcare Interoperability Resources (FHIR) framework.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Research firm KLAS released a new report on electronic medical record (EMR) usability and product performance over the past two years, revealing how physicians and other users rate various vendors and solutions. The report, entitled “Physician Leadership Weighs In on Acute Care EMR Usability” compares usability performance in the acute care EMR market segment.
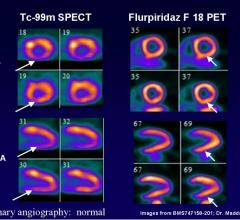
Results from Lantheus Medical Imaging Inc.’s first Phase 3 study of flurpiridaz F 18 for myocardial perfusion imaging (MPI) were presented at the International Conference on Nuclear Cardiology and Cardiac CT (ICNC12) in Madrid, Spain. The oral presentation was made by Jamshid Maddahi, M.D., professor of medicine (cardiology) and molecular & medical pharmacology (nuclear medicine), David Geffen School of Medicine at UCLA, in the “Important Clinical Trials and Registries in Nuclear Cardiology” session of ICNC12.

A large-scale, multicenter study has shown that therapeutic hypothermia does not improve survival rates or reduce brain injury in infants and children with out-of-hospital cardiac arrest more than normal temperature control.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Thoratec Corp. announced results from the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients). For the primary endpoint, a composite of survival and functional improvement, ROADMAP demonstrated a statistically significant benefit of HeartMate II LVAD support relative to optimal medical management in ambulatory New York Heart Association (NYHA) Class IIIB/IV (INTERMACS profile 4-7) heart failure patients. One-year study results were presented during the 35th Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation (ISHLT 2015) in Nice, France.
Boston Scientific Corp. is taking a new approach to evaluate the performance of the Vessix Renal Denervation System, initiating a study to isolate the effects of the therapy in patients with high blood pressure.
Sunshine Heart Inc. announced that the U.S. Food and Drug Administration (FDA) has reviewed the company’s submission regarding the pause of the COUNTER HF U.S. pivotal study. The FDA has requested minor protocol changes be submitted in order to receive approval to resume patient enrollment.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
There have been several technology advances in PET/CT (positron emission tomography/computed tomography) systems recently introduced by vendors. On the show floors of the 2014 Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting and the 2014 Radiological Society of North America (RSNA) meeting, Philips, Toshiba and GE Healthcare introduced new PET/CT systems.
It’s the invisible elephant in radiology — radiation exposure. As physicians, we know all too well that exposure to radiation should be limited to the least amount possible. We have all learned about the ALARA principle for keeping exposure low. We also take precautions on our patients’ behalf and educate them. But what about us? The physicians and technical staff who treat patients every day cannot avoid radiation exposure entirely. However, there is growing evidence that not only is radiation exposure detrimental to our health, but also many long-term cumulative effects are still unknown.
Based on its ongoing analysis of the medical imaging market, Frost & Sullivan recognizes Toshiba America Medical Systems, Inc. with the 2015 North American Frost & Sullivan Award for Medical Imaging Company of the Year. The company has changed the way it approaches the imaging market by offering scalable, upgradable technologies and a patient-focused view of partnerships. This value-based approach positions the company for continued success in the medical imaging market by allowing it to re-orient many of its imaging customers away from a cyclical sales model and toward deeper, more renewable strategic partnerships.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
One life-threatening complication of lung cancer surgery is the formation of blood clots in the lungs (also called pulmonary embolism, PE) or in the legs (also known as deep vein thrombosis, DVT). Together, they would be defined as venous thromboembolic events (VTE). Several presentations at AATS 2015 shed new light on this serious problem. In the first prospective study of its kind, the incidence of VTE was found to be higher than previously reported, with a 5.4 percent VTE-specific mortality rate. Of concern to clinicians, most events were asymptomatic and occurred after patients were discharged from the hospital. The second report highlights the importance of screening for VTEs, especially since the majority of lower extremity VTEs found after pneumonectomy would have gone undiagnosed and untreated without screening. The third report describes a risk assessment tool for VTEs that is applied for the first time to predict an individual’s risk of VTEs after lung cancer surgery, which can help clinicians decide whether prolonged anti-clotting therapy is warranted.
The Society for Cardiovascular Angiography and Interventions (SCAI) announced the following late-breaking clinical trials to be presented at its 2015 Scientific Sessions, May 6-9 in San Diego.
AstraZeneca announced that the U.S. Food and Drug Administration (FDA) has accepted a supplemental new drug application (sNDA) and granted Priority Review for Brilinta (ticagrelor) tablets for patients with a history of heart attack. The sNDA is based on the results of the PEGASUS-TIMI 54 study, a large-scale outcomes trial in more than 21,000 patients that investigated ticagrelor tablets plus low dose aspirin, compared to placebo plus low dose aspirin, for the chronic secondary prevention of atherothrombotic events in patients who had experienced a heart attack one to three years prior to study enrollment. The Prescription Drug User Fee Act goal date will be in the third quarter of 2015.


 May 05, 2015
May 05, 2015