The biggest technology news coming out of the 2015 American College of Cardiology (ACC) annual scientific sessions involved the overwhelmingly positive late-breaking trial data for transcatheter aortic and mitral valve repair. ACC dedicated an entire day of late-breaking trial presentations to this new technology, highlighting its importance in how cardiology will be changing in the coming years, moving from invasive surgical repairs to minimally invasive percutaneous therapies. There were reservations a couple years ago among most cardiologists that transcatheter procedures would not live up to the durability and outcome standards set by surgical valve replacement, but data continue to show it is comparable, and in some cases, better.
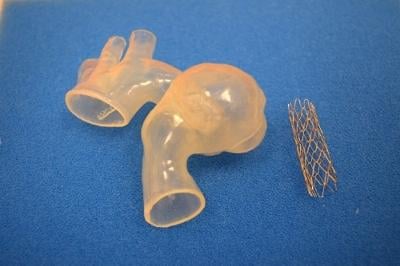
Seventeen-year-old Ariana Smith recently became the first patient at the Children's Hospital of Michigan, and the first in Michigan, to benefit from a revolutionary 3-D printed heart model to treat a very large, complex aortic aneurysm.
The results of a blood test done immediately after heart surgery can be a meaningful indicator of postoperative stroke risk, a study by researchers at Wake Forest Baptist Medical Center has found.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic announced Japanese regulatory approval for the self-expanding transcatheter CoreValve system for patients with severe aortic stenosis (AS). Japanese regulatory authorities granted approval of the CoreValve system for transcatheter aortic valve implantation (TAVI) based on data from the CoreValve U.S. Pivotal Trials and the Medtronic CoreValve Japan Trial, which is the first study to evaluate a self-expandable transcatheter valve in the Japanese patient population.
SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct an Investigational Device Exemption (IDE) clinical study on the effective use of its 50cc SynCardia temporary Total Artificial Heart.
Medtronic plc announced the initiation of the SPYRAL HTN Global Clinical Trial Program, a phased clinical program studying renal denervation in uncontrolled hypertension. This announcement follows investigational device exemption (IDE) approval by the U.S. Food and Drug Administration (FDA).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
UHC has released the findings of a new study that evaluated the use of medical devices based on cost and quality outcomes. The goal of the study was to uncover several trends related to high-priced physician preference items (PPIs). Utilization of PPIs — which can account for 30 to 40 percent of a hospital's supply expenses — at 10 academic medical centers, focusing on procedures utilizing orthopedic implants, coronary and peripheral stents and cardiac valves.
According to information technology (IT) company Logicalis US, managing a high volume of patient images in varying formats requires development of a comprehensive enterprise imaging strategy that addresses five core components while remaining vendor-neutral.
Mobile Heartbeat announced it will be demonstrating its MH-CURE communication and workflow smartphone application at the 2015 Healthcare Information and Management Systems Society (HIMSS) annual conference, April 13-15, 2015, in Chicago.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The physician-patient relationship still plays a primary role in the overall patient experience despite increasing use of technology, according to a survey conducted by Nuance Communications Inc. The 3,000-person survey — conducted in the United States, United Kingdom and Germany — found that 97 percent of people agreed with this sentiment.
A new KLAS report on business intelligence (BI) found that healthcare organizations are looking for BI vendors with industry expertise and content to handle the growing complexities of data management. The report — entitled Health Analytics 2015: Moving toward the Continuum of Care — stresses that in-organization data is only a part of the picture and that data aggregation capabilities are more important than ever before.
NavisHealth Solutions Inc. announced the general availability of its mobile patient engagement platform, Engage.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The U.S. Food and Drug Administration (FDA) expanded the approved use of the CoreValve self-expanding transcatheter aortic valve replacement (TAVR) system to treat certain patients who have previously had a tissue aortic valve replacement and are in need of a second one. This is the first transcatheter heart valve approved in the United States for valve-in-valve procedures in both high and extreme risk patients who have limited options or may otherwise go untreated.
The Medicines Company announced that the European Commission has granted marketing authorization for two acute care products – Kengrexal (cangrelor) and Raplixa (sealant powder). These approvals follow the issuance of positive opinions in January by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA).
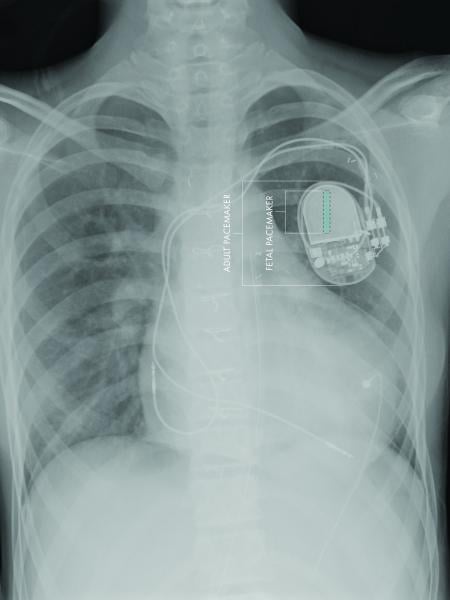
A team of investigators at Children’s Hospital Los Angeles and the University of Southern California have developed the first fully implantable micropacemaker designed for use in a fetus with complete heart block. The team has done preclinical testing and optimization as reported in a recent issue of the journal Heart Rhythm.

 April 02, 2015
April 02, 2015











