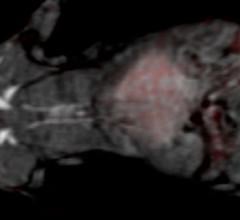
A sticky, protein-rich gel created by Johns Hopkins researchers appears to help stem cells stay on or in rat hearts and restore their metabolism after transplantation, improving cardiac function after simulated heart attacks, according to results of a new study.
September 29, 2015 — A new report from the American Heart Association (AHA) projects that the number of Americans ...
The Innovation Institute announced that Boston Scientific has signed an agreement to become the founding medical device sponsor of the institute’s Innovation Lab in Newport Beach, California.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
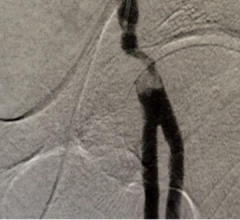
New 12-month clinical trial outcomes assessing the safety and performance of the Boston Scientific Eluvia drug-eluting vascular stent system reflect a primary patency rate of more than 96 percent.
Researchers have discovered how to predict some cardiac arrhythmias several steps before they even occur. It’s a finding that could lead to an improved cardiac device, with equipment designed to detect when arrhythmias are about to occur and then act to prevent them.
The Icahn School of Medicine at Mount Sinai has launched the international TWILIGHT clinical trial to test the safety and effectiveness of treating coronary stent patients with ticagrelor alone instead of combining it with aspirin, which is the current standard of care.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medisafe, an mHealth platform for medication management, announced the results of its blood pressure study, which showed app users with stage 1 or stage 2 hypertension decreased their systolic blood pressure by 17.7 and 25.3 mmHg, respectively, within 30 days.
At the 2015 IEEE High Performance Extreme Computing Conference (HPEC ‘15), ContextVision presented new results of research conducted with Texas Instruments (TI) and High Performance Consulting. The study outlines the achievement of low latency, reduced memory footprint and low power consumption through data streaming, specifically as a modification to portable ultrasound imaging devices.
The American College of Cardiology (ACC) launched two new clinical registry programs in August to track real-world outcomes for the treatment and stroke prevention of patients with atrial fibrillation.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
SHAPE, The Society for Heart Attack Prevention and Eradication, met with an international contingent of leading cardiologists and researchers to mark a decade of progress and determine the way forward. The SHAPE Trial Advisory Meeting took place Aug. 21-23, 2015, in Los Angeles.
Biotronik announced the publication of NORDIC ICD (NO Regular Defibrillation testing In Cardioverter-Defibrillator Implantation) trial results in the European Heart Journal. The company-sponsored, randomized, controlled trial contributes to a growing body of clinical evidence suggesting that defibrillation (DF) testing during routine first implantable cardioverter-defibrillator (ICD) implantation on the left side does not lead to significant clinical benefits. These results also demonstrate the shock efficacy of Biotronik ICDs in a large, real-world population.
The U.S. Food and Drug Administration (FDA) announced it is establishing an advisory committee focused on patient engagement. The Patient Engagement Advisory Committee will provide advice on complex issues relating to medical devices, the regulation of devices and their use by patients to the FDA Commissioner.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
A new guideline aimed at helping clinicians treat patients with supraventricular tachycardia, or a rapid heart rate, was released Sept. 23 by the American College of Cardiology, American Heart Association and Heart Rhythm Society.
CeloNova BioSciences Inc. announced that it has received conditional approval to start an investigational device exemption (IDE) trial to study the Cobra PzF coronary stent system in patients at high risk of bleeding.
September 23, 2015 — New research describes a method, tested in rats, that may someday allow healthcare providers to ...


 September 29, 2015
September 29, 2015