Ischemic heart failure from previous heart attacks and coronary artery disease is the leading cause of death in the world, affecting more than 12 percent of the world’s population, according to the World Health Organization. Stem cell therapy has been conducted to try to repair heart damage from ischemic heart failure, but in previous studies, the two types of stem cells (autologous bone marrow derived mesenchymal cells [MSCs] and endomyocardial biopsy derived c-kit+ Cardiac Stem Cells [CSCs]) have been used in separate trials. In a first-in-the-world study, the Minneapolis Heart Institute Foundation (MHIF) is about to begin the CONCERT study, led by Principal Investigator Jay Traverse, M.D. The study will use MSCs and CSCs together to learn if the combination would be more successful than using either alone based on pre-clinical studies in swine demonstrating an enhanced synergistic effect of the combination.
January 10, 2017 — Teleflex Inc. announced that its Arrow VPS Rhythm Device with optional TipTracker technology has been ...
Effective July 1, 2017 for Medicare heart attack patients, the the Centers for Medicare and Medicaid Services (CMS) will ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
BioCardia Inc. announced in December the issuance of United States Patent No. 9,517,199 relating to a method of delivering cells to patients who have chronic myocardial infarcts. This new patent follows United States Patent No. 9,504,642, issued to BioCardia two weeks prior.
Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo. The Duo is the next generation of the G-Arm, with major improvements including axial tilt and greater table access.
Lumedx Corp. announced at the end of November that two Marshall Medical Centers hospitals have gone live with the first phase of a cardiovascular information system (CVIS) deployment. The two hospitals now using Lumedx CVIS software are Marshall Medical North in Guntersville, Ala.; and Marshall Medical South in Boaz, Ala.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Arterys has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Arterys Cardio DL application. Arterys Cardio DL is the first technology to be cleared by the FDA that leverages cloud computing and deep learning in a clinical setting.

January 9, 2017 — The U.S. Food and Drug Administration (FDA) issued a safety communication today concerning patient ...
January 6, 2017 — SentreHeart Inc. announced that it has completed the Stage I enrollment milestone in the aMAZE Trial ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
ITN and DAIC Editor Dave Fornell takes a tour of some of the most innovative new technologies being displayed on the ...
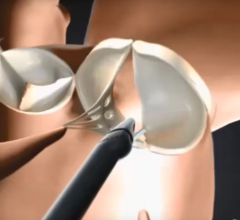
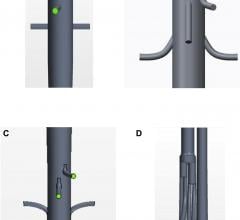
The RECHORD trial allows cardiovascular surgeons to replace damaged string-like tendons, called chordae, through a small incision while the heart is beating. The trial compares outcomes from this minimally invasive procedure to traditional open-heart mitral valve surgery, which requires the chest to be cracked. Specialized imaging is used to place the artificial chordae. PinnacleHealth is one of only 20 sites in the nation and the only hospital in Pennsylvania selected to participate in the trial. Up to 450 patients will be enrolled into the randomized trial.
Toshiba Medical announced in November that its Vantage Titan 1.5T/cS Edition magnetic resonance imaging (MRI) system with M-Power V3.6 software received U.S. Food and Drug Administration (FDA) clearance at the 2016 annual meeting of the Radiological Society of North America (RSNA). The new system retains all the patient-friendly features of the Vantage Titan 1.5T MR with added technology to simplify complex cardiac exams.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
January 5, 2017 — Whether patients with mechanical heart valves and stents must take blood thinners depends on how ...
Too little sleep takes a toll on your heart, according to a new study presented at the 2016 annual meeting of the Radiological Society of North America (RSNA), Nov. 27-Dec. 1 in Chicago.
January 5, 2017 — A new report published by Allied Market Research forecasts that the global thrombectomy devices market ...

 January 10, 2017
January 10, 2017