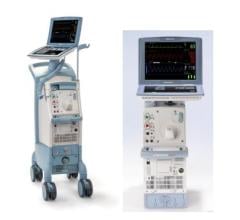
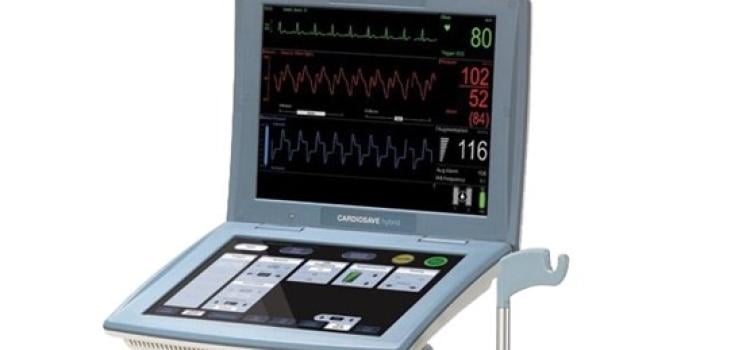
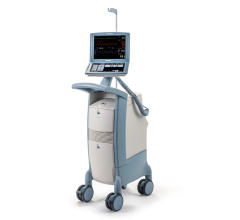
May 8, 2024 — The US Food and Drug Administration (FDA) is alerting health care providers and facilities about our continued safety and quality concerns with the following Getinge/Maquet ...
Intra-Aortic Balloon Pumps (IABP)
This channel includes news and new technology innovations for intra-aortic balloon pumps (IABP). IABPs are a type of hemodynamic support device that is inserted into the aorta to help augment the flow of blood with a pulsing balloon that pushes the blood volume out of the the aorta to help perfuse organs and tissues.
May 8, 2024 — The US Food and Drug Administration (FDA) is alerting health care providers and facilities about our ...
August 14, 2023 — The FDA has announced that Datascope/Maquet/Getinge is recalling the Cardiosave Hybrid and Rescue ...
June 22, 2023 — Lankenau Medical Center, part of Main Line Health, has performed the first minimally invasive catheter ...
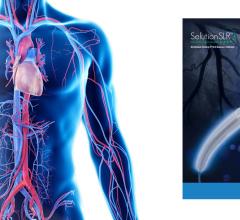
May 30, 2023 — Twelve-month results from the SELUTION SFA trial have been presented for the first time at the Japan ...
May 17, 2023 — Edwards Lifesciences announced that new data from the Benchmark Registry in Europe demonstrated the ...
May 4, 2023 — The first US patient has been enrolled in the SELUTION4SFA Sirolimus DEB study by Dr. Arthur Lee at the Ca ...
March 31, 2023 — According to statement issued by the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
March 21, 2023 — Procyrion, Inc., a medical device company dedicated to improving outcomes for patients with cardiac ...
March 20, 2023 — According to a statement issued by the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
March 14, 2023 —MedAlliance has announced enrollment of over 1,000 patients in its ground-breaking SELUTION DeNovo coron ...
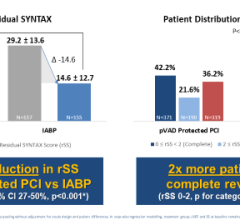
February 24, 2023 — Abiomed, Inc., part of Johnson & Johnson MedTech[1], announced that results from a study on Impella ...
January 27, 2023 — The first US patient has been enrolled at Medstar Washington Hospital Center in the SELUTION4ISR ...
January 25, 2023 — According to a new release from the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
January 12, 2023 — Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional FDA ...

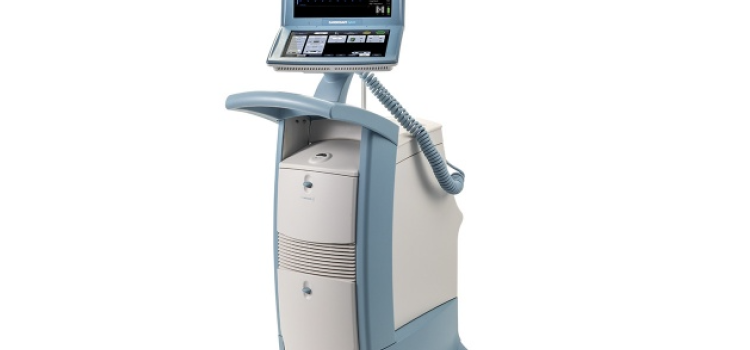


 May 08, 2024
May 08, 2024