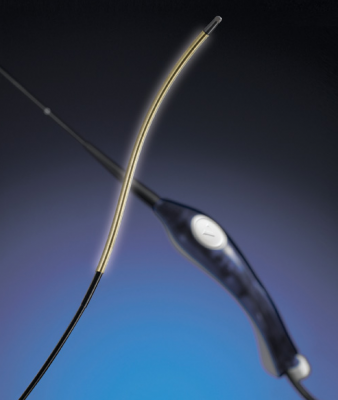
February 6, 2012 — Vascular Solutions Inc. announced it is marketing a reprocessing service for the ClosureFAST radiofrequency ablation catheter in the United States. The ClosureFAST catheter, manufactured and marketed by VNUS Medical Technologies Inc. — a division of Covidien — is widely used for performing endovenous therapy for the treatment of varicose veins. Vascular Solutions' reprocessing service is designed to help physicians reduce medical waste and lower their costs.
Vascular Solutions offers the reprocessing service under contract with Northeast Scientific Inc. (NES), an established third-party reprocessor of medical devices. NES received 510(k) clearance from the U.S. Food and Drug Administration (FDA) Nov. 30, 2011 for reprocessing the ClosureFAST catheter.
"Reprocessing has proven to be a safe and effective way for hospitals and clinics to cut down on medical waste and reduce operating costs. As a result, the vast majority of U.S. hospitals are now contracting with third-party reprocessors for a wide variety of medical devices," said Howard Root, CEO of Vascular Solutions. "Reprocessing has become one of the fastest-growing segments of the medical device industry, and we are very pleased to be entering this market in collaboration with NES for the ClosureFAST catheter, which we estimate to be generating in excess of $70 million in annual sales in the U.S."
NES has granted Vascular Solutions the exclusive right through Dec. 31, 2016 to market the ClosureFAST reprocessing service to hospitals and clinics within the United States through Vascular Solutions' direct sales force. Vascular Solutions has already begun to take orders for the ClosureFAST reprocessing service and NES has begun to receive used ClosureFAST catheters from several of these customers for reprocessing.
Hospitals and clinics that subscribe to the service send their used ClosureFAST catheters directly to NES for reprocessing. Upon completion of reprocessing, customers receive those same catheters back, ready for a second use. NES's validated reprocessing system for the ClosureFAST catheters involves multiple stages, including decontamination, cleaning, drying, packaging, labeling, sterilization, and biological quarantine testing. As part of the process, each catheter is subjected to function testing and undergoes multiple inspections to ensure that quality standards are met.
"Regulators hold third-party processors such as NES to extremely high standards for safety and effectiveness, and we are proud that we have successfully completed 46 different tests to document that our reprocessed vein ablation catheters meet the industry's highest performance standards," said Craig Allmendinger, CEO of NES. "We know that Vascular Solutions shares our commitment to patient safety and product performance, and we are happy to be working with a company with a nationwide direct sales force that is already experienced with the needs of the vein therapy clinical community."
For more information: www.vasc.com, www.mdreprocess.com


 February 06, 2026
February 06, 2026 









