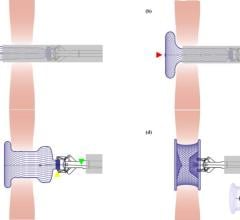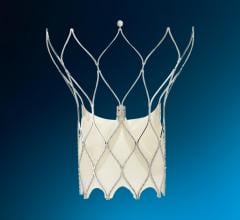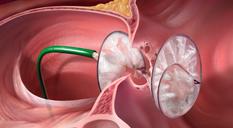
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder with a modified catheter delivery system indicated for the transcatheter closure of atrial septal defect (ASD), providing a percutaneous ASD closure solution for very young patients.
The GORE HELEX Septal Occluder is a permanently implanted prosthesis that uses ePTFE, a biocompatible material that allows tissue ingrowth, to seal the defect.


 June 20, 2024
June 20, 2024