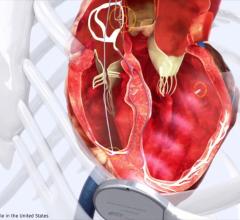
February 8, 2010 – The FDA cleared the Sorin Paradym CRT Model 8750, a cardiac resynchronization therapy defibrillator (CRT-D), which features a new battery technology that delivers 37 joules, one of the highest energy levels of any implantable cardiac defibrillator (ICD).
Paradym offers consistent charge times throughout the life of the device (10s at beginning of life, 13s at elective replacement indicator - ERI), improved longevity, and a six-month ERI to end of service (EOS) period.
The CRT is designed to allow more flexibility in the management of cardiac resynchronization and anti-tachyarrhythmia therapy in heart failure patients. Brady-tachy overlap (BTO) is designed to unlock pacing and detection to ensure delivery of resynchronization therapy at high pacing rates during exercise without compromise on the management of slow ventricular tachycardias (VTs).
“I’m impressed with the Sorin technology”, said Dwight Reynolds, M.D., chief of the cardiovascular section at the University of Oklahoma Health Sciences Center in Oklahoma City, Okla., who performed the first U.S. implant of Paradym CRT. “They have managed to pack a lot of power into a small can without compromising on features, good charge times or longevity. I especially like the six months longevity post-ERI and the PARAD+ discrimination algorithm to minimize inappropriate shocks…that is extremely important to both my patients and myself.”
Paradym CRT, at 34 cc and 11 mm wide, also features the PARAD+ detection algorithm, which has specificity in discriminating ventricular arrhythmias. Studies have demonstrated that the absolute risk of experiencing an inappropriate shock has been observed to be only 5 percent.
For more information: www.sorin.com


 July 21, 2025
July 21, 2025