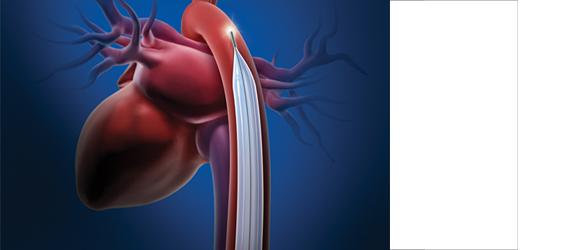
June 3, 2011 -- Maquet Cardiovascular announced that the U.S. Food and Drug Administration (FDA) has granted the company 510(k) clearance to market its CardioRoot aortic graft in the United States. A one-piece design, aortic root graft, CardioRoot will be used by vascular and cardiovascular surgeons to surgically repair or replace diseased and damaged aortae. CardioRoot is now commercially available in the United States. CardioRoot’s design mimics the anatomy of the patient’s native aortic root, including the clinically important natural sinuses of valsalva, which supply blood to the heart’s own arteries. CardioRoot’s anatomically correct shape allows easy sewing of valve remnants or a prosthetic valve within the tube, thereby avoiding potential bleeding while shortening surgical procedure time. “The CardioRoot graft is an important option for aortic root repair. The graft wall is supple, allowing better approximation with tissue. The unique properties of the graft material provide ease of suturing and superb predictability of the final geometry,” said Marc W. Gerdisch, M.D., chief of cardiothoracic surgery at Franciscan St. Francis Heart Center in Indianapolis, and a clinical assistant professor of cardiothoracic surgery at Loyola University Medical Center. Dr. Gerdisch is the first cardiothoracic surgeon in the United States to use CardioRoot on a critically ill patient. “The improved hemostasis is an added advantage of CardioRoot for complex aortic root reconstructions, substantially contributing to a dry surgical field at the end of the surgery." For more information: www.maquet.com


 February 06, 2026
February 06, 2026 









