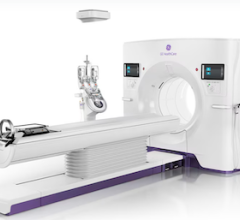
December 6, 2012 — Neusoft Medical Systems Co. Ltd., a wholly owned subsidiary of Neusoft Corp., announced this week that its NeuViz 64 multi-slice computed tomography (CT) scanner has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
The NeuViz 64 design delivers low-dose scanning, high patient throughput, ease of use, performs advanced cardiac imaging and provides a wide variety of clinically relevant post-processing and diagnostic techniques. The Quad-Sampling Technology can improve resolution, reduce artifact and extend scanning ranges. ClearView Reconstruction removes noise while preserving details, potentially providing low-dose image quality that is superior to that of full-dose images. Low-dose designs applied in NeuViz 64 include 240-degree exposure, pediatric protocols and electrocardiogram (ECG) dose modulation. A powerful workstation (AVW) can offer a full range of clinical applications, and optimized intuitive workflow guides technologists to use the system easily and quickly.
Neusoft Medical continues to expand its global presence around the world. It has achieved ISO9001 quality system certification for all products, among which CT, magnetic resonance imaging (MRI), X-ray, diagnostic ultrasound and positron emission tomography (PET) products have been certified by CE and the FDA. Beside the 64 multi-slice CT, Neusoft Medical is innovating 64+ slice CT at the meantime, focusing on lower dose and better image for quality diagnosis.
The scanner also comes in the NeuViz 64 Cardiac configuration for specialized cardiac CT angiography imaging.
For more information: www.medical.neusoft.com/en/

 March 28, 2025
March 28, 2025