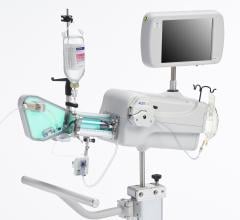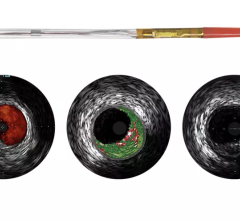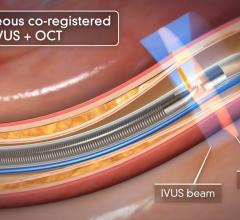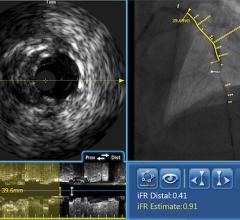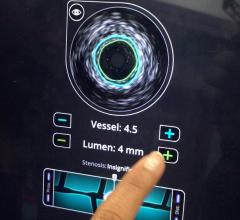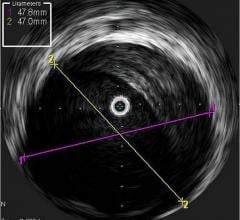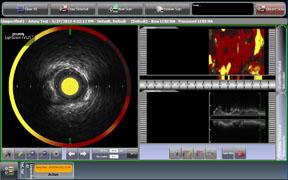
September 1, 2010 – The first catheter to combine near-infrared (NIR) spectroscopy with intravascular ultrasound (IVUS) to characterize coronary plaque was cleared by the U.S. Food and Drug Administration (FDA). InfraReDx Inc. received FDA 510(k) clearance to market the LipiScan IVUS Coronary Imaging System. It is designed to help cardiologists identify and characterize the plaques that complicate stenting and are associated with acute coronary events. The NIR spectroscopy identifies the chemical content of the plaques. The IVUS provides an image of plaque structure and stent features. The company expects to conduct a broad commercial launch of the system within the United States by the end of 2010. It anticipates regulatory approval and launch in Europe during 2011. InfraReDx's first-generation LipiScan NIR system is already in use at about 25 U.S. cardiac centers and is the only FDA-cleared device for the detection of lipid core coronary plaques (LCP). LCP is a fatty coronary artery plaque known to complicate stenting and suspected to cause most heart attacks following stenting. The addition of IVUS imaging enhances the value of LipiScan by providing visualization of the coronary artery lumen, assessment of the structural features of coronary plaque, and identification of proper stent sizing and expansion. The LipiScan IVUS provides a grayscale IVUS image of the coronary artery along with a complete and co-registered chemogram – a map of LCP within the imaged vessel. It helps physicians identify lipid-core plaques, the degree of stenosis, reference vessel diameter, plaque burden and stent expansion and apposition. “The LipiScan IVUS System significantly advances the field of intracoronary imaging,” said James Goldstein, M.D., director of cardiac research and education for the William Beaumont Hospital in Michigan. “We are eager to bring the system to the William Beaumont Hospital, where we are already successfully employing the LipiScan NIR system and gleaning key insights from the chemogram information it provides. The inclusion of plaque structure data via LipiScan IVUS further elevates the system's practical clinical utility for characterizing coronary lesions and identifying lipid core plaque. This information can better inform technique and treatment decisions for my patients today, and, in the future, holds the promise to help prevent heart attacks and sudden death." Goldstein has been involved in vulnerable plaque-related research for more than 10 years, and currently serves as a consultant to InfraReDx as well as other medical device and imaging technology companies. “Patients are increasingly presenting to the cath lab with complex lesions, such as left main disease, multi-vessel disease and bifurcation lesions,” said David Rizik, M.D., medical director of invasive cardiology at Scottsdale Healthcare Shea Medical Center. “In these patients, angioplasty guided by angiography alone is inadequate to prevent potential complications such as restenosis, stent thrombosis and periprocedural myocardial infarction.” For more information: www.infraredx.com


 September 18, 2025
September 18, 2025