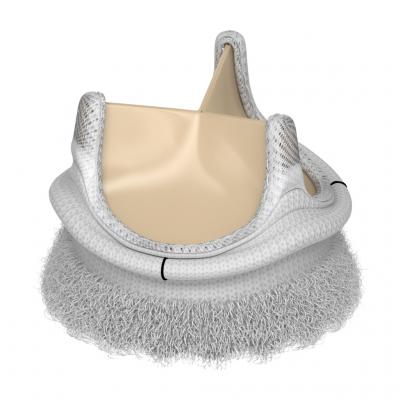
The Edwards Intuity Elite valve system uses a balloon expandable skirt to anchor the valve rather than traditional sutures.
August 15, 2016 – Edwards Lifesciences announced the U.S. Food and Drug Administration (FDA) approved of the advanced Edwards Intuity Elite valve system, a rapid deployment device for surgical aortic valve replacement. Built on the Perimount tissue valve platform and incorporating innovations from transcatheter heart valves, the the new valve is designed to facilitate minimally invasive surgery and streamline complex aortic valve replacements, thereby offering a cutting-edge treatment option for patients with aortic valve disease. The device is now commercially available.
“Many patients with aortic stenosis have more than one type of heart disease, most commonly coronary artery disease. The Edwards Intuity Elite valve system enables surgeons to streamline concomitant procedures, which can be beneficial for patients undergoing these longer, more complex surgeries,” said Kevin Accola, M.D., program director, Valve Center for Excellence at the Florida Hospital Cardiovascular Institute in Orlando, Fla.
Mubashir A. Mumtaz, M.D., FACS, FACC, chief of cardiothoracic surgery and surgical director of the structural heart program at PinnacleHealth in Harrisburg, Pa., said, “Patients request less invasive surgical options, and the Edwards Intuity Elite valve system meets this need by facilitating multiple approaches to minimally invasive operations, which can mean less trauma, early recovery and decreased need for blood transfusions for many patients.”
FDA approval of the Edwards Intuity Elite valve system was supported by data from the TRANSFORM clinical trial, which treated 839 patients in 29 centers in the U.S. The results of this trial were presented recently during a late-breaking session at the American Association for Thoracic Surgery’s (AATS) 96th annual meeting. The data showed that, at one year, the Edwards Intuity valve system is safe and effective and may reduce cross-clamp time and cardiopulmonary bypass time, compared to times recorded in the Society of Thoracic Surgeons' (STS) Adult Cardiac Database. This may provide patient benefits such as decreased mortality and morbidity, less time in an intensive care unit and reduced total hospital stay. Overall, the New York Heart Association (NYHA) Functional Classification, which categorizes patients into one of four groups based on their heart failure symptoms and physical limitations, improved in 73.1 percent of patients at one year.
The device was approved for commercial sale in Europe in 2014.
For more information: www.edwards.com


 February 06, 2026
February 06, 2026 









