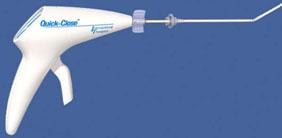
Quick-Close Suture Applier
April 26, 2010 – A vascular closure device using a single suture fitted with stainless steel rings was recently cleared by the U.S. Food and Drug Administration (FDA)
The Quick-Close Vascular Suturing System from Interventional Therapies LLC ?is used to stop the bleeding of a puncture site following an interventional procedure using the femoral artery. It consists of the Quick-Close Suture Applier and the Quick-Ti Cinch Applier. The suture applier has a pistol grip shape and contains a single strand of an artificial suture (polybutester Novafil) fitted with stainless steel rings. The clinch applier has a syringe-type shape with an extended handle.
The suture applier is used to gain access to the puncture site. Using manual control, and relying on the sense of touch, the device is used to place one end of the suture through one edge of the arterial wound. The suture applier is then repositioned and the other end of the suture is inserted through the opposite edge of the wound site. As the suture applier is withdrawn from the wound, a 24-inch length of suture is forced out of its housing. The suture applier places a single stitch of a nonabsorbable, single thread suture to the wound site. The cinch applier gathers the two ends of the suture and then it places a stainless steel ring that acts in place of a knot to secure the suture in a closed position to stop the bleeding.
It is designed for use in vascular punctures with diameters of 4 mm or larger.
The device allows patients to become mobile sooner than is possible with standard compression methods.
For more information: www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances


 October 27, 2021
October 27, 2021 







