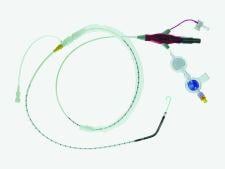
The Abiomed Inc. Impella 2.5 cardiac assist device was FDA cleared in 2008 for use for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the U.S.
The Impella 2.5 is inserted percutaneously in the catheterization laboratory via the femoral artery into the left ventricle. Up to 2.5 liters of blood per minute are delivered by the pump from the left ventricle into the ascending aorta, providing the heart with active support in critical situations.


 June 19, 2024
June 19, 2024 









