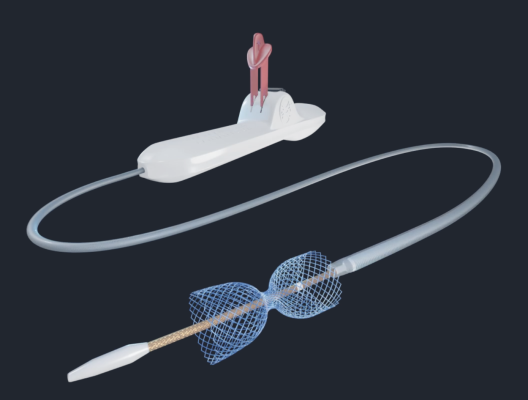
VahatiCor, Inc., a med tech company dedicated to helping patients with persistent ischemic heart disease, has announced the treatment of the first patient with the A-FLUX Reducer System, a treatment for patients with angina or chest pain. Image courtesy: VahitiCor
March 11, 2024 — VahatiCor, Inc., a med tech company dedicated to helping patients with persistent ischemic heart disease, has announced the treatment of the first patient with the A-FLUX Reducer System, a treatment for patients with angina or chest pain.
Angina is often caused by reduced blood flow to the heart. This condition can occur in people with or without significant blockages in their large coronary arteries. It is frequently accompanied by decreased blood flow in the very small blood vessels branching off the coronary arteries, a condition known as coronary microvascular dysfunction or microvascular angina.
The A-FLUX Reducer implant is placed in the coronary sinus, the largest vein in the heart, and is designed to provide more blood flow to the ischemic portion of the heart and improve patient symptoms and quality of life, according to a written statement issued by VahatiCor.
The A-FLUX Reducer System is an investigational medical device that is not available for commercial distribution.
The first patient received the A-FLUX Reducer implant through the Special Access Program (SAP) of Health Canada. A prospective multi-center clinical study of the device is scheduled to begin enrollment this year.
“We have many interventional or surgical revascularization options for patients with advanced large coronary artery disease. However, a significant proportion still suffer from angina. The A-FLUX Reducer could help to fill that therapeutic void. It was an honor to perform the first-in-human implant and the patient is doing great,” said Dr. Jean-Michel Paradis, specialist in coronary and structural interventional cardiology at Quebec Heart and Lung Institute, who co-treated the first patient.
“The A-FLUX Reducer System is a promising intervention with the potential to provide a predictable and low-risk treatment for a growing population of patients with angina symptoms that do not respond well to medicines and lifestyle changes,” added Dr. Can Manh Nguyen, interventional cardiologist who co-treated the first patient with Dr. Paradis.
More than 20 million people in North America and Europe have ongoing angina symptoms, according to the American Heart Association (AHA), and 4 million undergo invasive diagnostic angiograms per year.1-4
“While many interventions are available to treat the blockages in the large coronary arteries, very few options exist for an even larger group of patients with microvascular angina,” said Howard Edelman, CEO of VahatiCor. “We are encouraged by the positive early clinical experience with A-FLUX and are moving forward quickly to a larger study that will support the availability of the device for more patients,” Edelman added.
VahatiCor, Inc., based in Santa Clara, CA, is a member of the T45 Labs portfolio. T45 Labs is a specialized medical technology innovation company located in Santa Clara, Silicon Valley, CA. VahatiCor further noted that it is developing medical devices that address unmet clinical needs to elevate the standard of care for people with angina and other heart conditions.
For more information: www.vahaticor.com
Reference
1. Mozaffarian D, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016; 133:e38–e360
2. European Society of Cardiology, Disease of the smallest heart blood vessels is important global health problem. 27 May 2021.
3. Cook S, Walker A, Hügli O, Togni M, Meier B. Percutaneous coronary interventions in Europe: prevalence, numerical estimates, and projections based on data up to 2004. Clin Res Cardiol. 2007;96(6):375 382.
4. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886 895.


 February 06, 2026
February 06, 2026 









