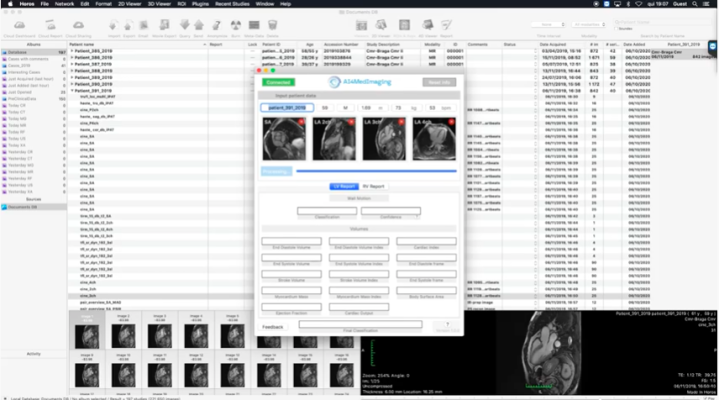
August 10, 2023 — Blackford, the pioneering strategic imaging AI platform and solutions provider, and AI4MedImaging, a cardiac MRI solution provider announce a partnership to make the AI4CMR solution available on the Blackford Platform. AI4CMR will enable Blackford customers to automatically segment and quantify cardiac MRI measurements.
"Blackford’s mission is to deliver AI that provides value to healthcare providers to increase efficiency and improves patient outcomes,” said Blackford CEO Ben Panter. “We are excited to increase our cardiac MRI solution offering on the Blackford Platform with AI4MedImaging.”
AI4CMR uses artificial intelligence to calculate, quantify, and analyse the left and right ventricle within 2 minutes, and produces a structured report to be extracted to any reporting system. It also provides a triage functionality for wall motion detection (this functionality is CE marked and FDA-pending).
“AI4MedImaging is thrilled to partner with Blackford and contribute to the democratization of Cardiac Magnetic Resonance. With AI4CMR, the first cardiac AI to receive CE and FDA approval, analysis is fast, accurate, made-easy and available at any location due to the web-based cloud technology used,” said AI4MedImaging’s CEO and MD Vítor Pereira.
For more information: www.blackfordanalysis.com


 January 15, 2026
January 15, 2026 









