July 21, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech,i announced that enrollment is complete in the SmartfIRE study designed to evaluate the safety and efficacy of its investigational THERMOCOOL SMARTTOUCH SF Dual Energy Catheter and investigational TRUPULSE Generator for the treatment of drug refractory symptomatic paroxysmal atrial fibrillation (AFib) during standard electrophysiology mapping and ablation procedures.[i] The catheter and the generator are fully integrated with the CARTO 3 Advanced 3D Imaging System.
SmartfIRE is a pivotal, prospective, multi-center, single-arm study evaluating Biosense Webster’s dual energy pulsed field (PF) and radiofrequency (RF) ablation system. Since the study’s commencement in February 2023, the trial has enrolled 149 patients with paroxysmal AFib across nine centers in Europe. Patients will be assessed for 12 months for safety and efficacy.1
“Biosense Webster’s dual energy ablation system has the potential to enable electrophysiologists to eliminate the need to exchange catheters and allow them to seamlessly switch the energy source, whether RF or PF, based on patient needs,” said Gediminas Račkauskas, M.D., Ph.D., Cardiac Electrophysiologist, Vilnius University Hospital Santaros Clinics, Lithuania.ii “Together with the CARTO 3 Mapping System that enables real-time catheter visualization, this system has the potential to drive enhanced safety, efficiency, and effectiveness of ablation procedures, while allowing doctors to utilize a familiar workflow.”
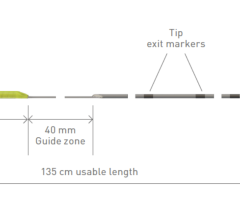
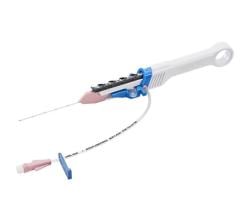
The THERMOCOOL SMARTTOUCH SF radiofrequency catheter has been available in Europe since 2016. It is the most commonly used ablation catheter in the world for RF ablation, and many electrophysiologists have a high degree of familiarity and confidence in using this tool for highly precise cardiac ablation procedures.[ii] The THERMOCOOL SMARTTOUCH SF Dual Energy Catheter with Contact Force Sensing Capability is an investigational steerable, multi-electrode luminal, irrigated catheter for PF and RF ablation. The investigational TRUPULSE Generator provides both the RF energy and the novel uni-polar, bi-phasic pulse field (PF) energy to the catheter through the toggling of the two energy sources on the generator monitor.[iii] The THERMOCOOL SMARTTOUCH SF Catheter is to be used in this investigational study with the TRUPULSE Generator, to transmit the PF or RF energy to the catheter tip electrode for cardiac ablation. The catheter and the generator are integrated with the CARTO™ 3 System. The TRUPULSE Generator is an investigational device and is not available for sale in the US or in Europe.
“Given the strong trust and familiarity physicians have with the THERMOCOOL SMARTTOUCH SF catheter and also industry interest in the potential of PFA, we saw a high level of excitement for participation in the SmartfIRE clinical trial,” said Jasmina Brooks, President, Biosense Webster, Inc. “This study will contribute to a growing body of evidence being developed by Biosense Webster to support the use of PFA as an additional option for AFib treatment, complementing our best-in-class RF ablation catheter portfolio.”
Catheter ablation is a minimally-invasive procedure performed by an electrophysiologist to treat heart rhythm disorders, including AFib, by interrupting irregular electrical pathways in the heart by delivering either heat (RF ablation) or cold (Cryoablation).[iv] PFA could represent a new approach to treating AFib, utilizing a controlled electric field to selectively ablate cardiac tissue that causes the irregular heart beat through a process called irreversible electroporation (IRE). Because the pulsed field energy is minimally thermal, IRE offers the potential to reduce the risk of damage to surrounding tissues including esophageal, pulmonary vein, and phrenic nerve injury.[v]
AFib is the most common type of cardiac arrhythmia and impacts nearly 37.5 million people worldwide,[vi] and 11 million people in Europe alone.[vii] Today, about 1 in 4 adults over the age of 40 are at risk for developing AFib.[viii] Despite these projections, many people are unfamiliar with AFib symptoms, available treatment options and the importance of early treatment to avoid disease progression.[ix]
In March 2023, Biosense Webster announced the first patients treated in the SmartfIRE study. The THERMOCOOL SMARTTOUCH SF Dual Energy Catheter and the TRUPULSE Generator are under development and not available for sale in any region of the world.
For more information: https://www.jnjmedtech.com/en-US/service/pulsed-field-ablation-evidence
References:
i Johnson & Johnson MedTech comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson’s MedTech segment.
ii Vilnius University Hospital Santaros Clinics, Lithuania, entered into a clinical trial agreement with Johnson & Johnson Medical NV/SA for its participation in the SmartfIRE Study. Dr. Gediminas Račkauskas serves as a study investigator and was not compensated for his contributions to this announcement.
[i] ClinicalTrials.gov. Safety and Effectiveness Evaluation of the THERMOCOOL SMARTTOUCH™ SF Catheter with the TRUPULSE™ Generator for treatment of Paroxysmal Atrial Fibrillation (PAF) (SmartfIRE). https://clinicaltrials.gov/ct2/show/NCT05752487.
[ii] Diagnostic and Interventional Cardiology. Patient Enrollment Completed in U.S. IDE Study of THERMOCOOL SMARTTOUCH SF Catheter. https://www.dicardiology.com/content/patient-enrollment-completed-us-ide-study-thermocool-smarttouch-sf-catheter.
[iii] ClinicalTrials.gov. A Study for Treatment of Paroxysmal Atrial Fibrillation (PAF) by Pulsed Field Ablation (PFA) System With Irreversible Electroporation (IRE) (inspIRE). https://clinicaltrials.gov/ct2/show/NCT04524364.
[iv] Cleveland Clinic. Catheter Ablation. https://my.clevelandclinic.org/health/treatments/16851-catheter-ablation Accessed July 7, 2023
[v] Reddy VY, Neuzil P, Koruth JS, et al. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. JACC 2019;74(3)315-326.
[vi] Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021 Feb;16(2):217-221. doi: 10.1177/1747493019897870. Epub 2020 Jan 19. Erratum in: Int J Stroke. 2020 Jan 28;:1747493020905964. PMID: 31955707.
[vii] Global Burden of Disease Collaborative Network (2016) Global Burden of Disease Study 2016 (GBD 2016) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2017.
[viii] Staerk L, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018 Apr 26;361:k1453.
[ix] Kuck et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23(3)362-369. PMID: 33330909


 January 29, 2026
January 29, 2026