Getty Images
June 21, 2023 — Anumana, Inc., a leading AI-driven health technology company, has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its ECG-AI algorithm designed to aid the early identification of cardiac amyloidosis, a life-threatening, rare, underdiagnosed cause of heart failure. This breakthrough device designation was granted to provide patients and healthcare providers timely access to the algorithm, subject to approval.
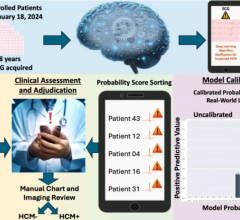
Anumana previously announced a multi-year research agreement with Pfizer to develop ECG-AI solutions to aid in early identification of cardiac amyloidosis. The Breakthrough Device Designation recognizes the significant potential of this algorithm, and the designation is a key regulatory milestone under the agreement, providing an expedited pathway for regulatory review1. Anumana plans to conduct a retrospective clinical validation trial and pursue De Novo classification for the algorithm as Software as a Medical Device (SaMD) and aims to gain regulatory approval for the algorithm for the detection of cardiac amyloidosis in the U.S., Europe, and Japan.
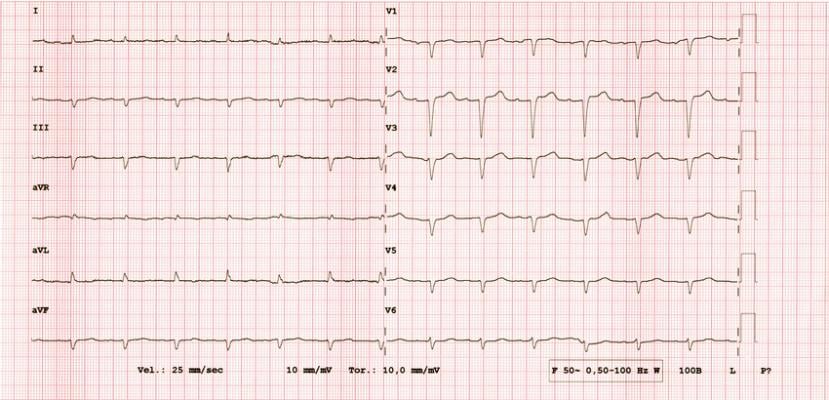
Cardiac amyloidosis is an underdiagnosed disease that often leads to heart failure.2 Early diagnosis is crucial to enable timely interventions that can improve outcomes.3 However, diagnosing this condition early can be challenging because of the rarity of the disease, which often presents with nonspecific signs and symptoms.4 While a standard ECG is obtained during the evaluation of these nonspecific symptoms, human interpretation of the ECG often misses subtle combinations of features that may indicate cardiac amyloidosis.5,6
Developed in collaboration with Mayo Clinic, Anumana’s ECG-AI algorithm aims to address this medical need with breakthrough technology that facilitates earlier identification of this underdiagnosed condition. Anumana is currently developing this algorithm as a SaMD, with the goal of integrating this solution into existing clinical workflows.
“The use of AI algorithms to identify subtle signals in ECGs that are imperceptible to humans stands to transform cardiovascular medicine by allowing us to detect disease early, in more easily treated stages, potentially avoiding serious consequences,” said Paul Friedman, M.D., Chair of the Department of Cardiovascular Medicine at Mayo Clinic in Rochester and Chair of Anumana’s Mayo Clinic Board of Advisors. “It is encouraging to see an algorithm that can aid in identification of cardiac amyloidosis receive Breakthrough Device Designation, as it recognizes the importance of having new tools to detect rare cardiac diseases in ways never before possible.”
Anumana, a portfolio company of nference was founded in 2021 in collaboration with the Mayo Clinic Platform and has since developed a robust pipeline of FDA-ready ECG-AI algorithms. These algorithms have been validated in over 75 peer-reviewed publications - including two novel, real-world prospective studies published in Nature Medicine and The Lancet. Anumana has received four Breakthrough Device Designations from the U.S. FDA for algorithms aimed at early identification of low ejection fraction, pulmonary hypertension, hyperkalemia, and now, cardiac amyloidosis.
“The ubiquitous nature of the painless, non-invasive Electrophysiology tests gives ECG-AI algorithms the potential to reach a larger number of patients earlier, something clinicians have long hoped for,” said Venky Soundararajan, PhD, Co-founder, and Chief Scientific Officer of Anumana and nference. “Receiving the FDA Breakthrough Device Designation for our Cardiac Amyloidosis ECG-AI Algorithm recognizes the significant potential of this tool to detect disease early.”
For more information: www.anumana.ai
References:
1 https://www.fda.gov/medical-devices/how-study-and-market-your-device/breakthrough-devices-program#s1
2 https://pubmed.ncbi.nlm.nih.gov/25758359/
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6823341/
4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5392416/
5 https://pubmed.ncbi.nlm.nih.gov/30126010/
6 https://academic.oup.com/ehjdh/article/3/2/238/6586624


 September 24, 2025
September 24, 2025