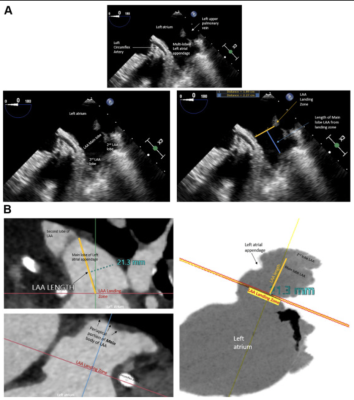
Transesophageal echocardiogram (TEE) and computed tomography angiography (CTA) images of the left atrial appendage (LAA) landing zone and definition of optimal LAA length measurements. (A) TEE images demonstrate the multilobed LAA that emanates from a larger body of the main lobe of the LAA. The landing zone is depicted by an orange line measured at the level of the circumflex artery. The length of the main lobe of the LAA is depicted by the blue line measured from the centroid of the LAA landing zone (orange line) to the center of the periapical region of the main body of the LAA. Care is taken to avoid measuring lengths to accessory LAA lobes as this may not accurately mimic the ability of current generation LAA occlusion (LAAO) devices to fully expand within very narrow portions of the distal lobes of the LAA. (B) CTA images demonstrate the multilobed LAA that emanates from a larger body of the main lobe of the LAA. The landing zone is depicted by a red line measured from the centroid of the LAA landing zone (demarcated by solid orange line), to the center of the periapical region of the main body of the LAA. Care is taken to avoid measuring lengths to accessory LAA lobes as this may not accurately mimic the ability of current generation LAAO devices to fully expand within very narrow portions of the distal lobes of the LAA.Image courtesy of jscai.org
March 28, 2023 — The Society for Cardiovascular Angiography and Interventions (SCAI) and the Heart Rhythm Society (HRS) released an updated expert consensus statement on transcatheter left atrial appendage closure (LAAC). SCAI and HRS prioritized the development of an updated consensus statement to provide recommendations on contemporary, evidence-based best practices for transcatheter LAAC focusing on endovascular devices.
Left atrial appendage closure is a minimally invasive procedure that is used to reduce the risk of stroke associated with atrial fibrillation. Atrial fibrillation is a common form of arrhythmia, a condition in which the heart beats out of rhythm. Atrial fibrillation (AF) is associated with a 4- to 5-fold increased risk of ischemic stroke and accounts for 25% of the 700,000 cerebrovascular accidents that occur in the United States annually.
Summary of Recommendations:
1. Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk who are not suited for long-term oral anticoagulation and who have adequate life expectancy.
2.1. Physicians performing LAAC should have prior experience, including ≥50 prior left-sided ablations or structural procedures and ≥25 transseptal punctures (TSPs). Interventional imaging physicians should have experience in guiding ≥25 TSPs before supporting any LAAC procedures independently.
2.2. For maintenance of skills, implanting physicians should perform ≥25 TSPs and >12 LAACs over each 2-year period.
2.3. New programs and implanting physicians early in their LAAC experience should have on-site cardiovascular surgery backup.
3. Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography is recommended before LAAC.
4. Intraprocedural imaging guidance with TEE or intracardiac echocardiography is recommended.
5. Technical aspects of the procedure, including venous access, anticoagulation, transseptal puncture, delivery sheath selection and placement, left atrial pressure measurement, and device deployment, should be performed in accordance with the labeling of each specific LAAC device.
6. Operators need to be familiar with avoidance, recognition, and management of procedural complications associated with LAAC.
7. Predischarge imaging should be performed with 2-dimensional transthoracic echocardiography to rule out pericardial effusion and device embolization.
8. Device-related thrombus should be treated with anticoagulation.
9. Routine closure of iatrogenic atrial septal defects associated with LAAC should not be performed.
10. The clinical impact and management of peridevice leaks are not fully understood, and all efforts should be made to minimize such leaks at the time of implantation.
11. Patients should be prescribed antithrombotic therapy with warfarin, direct oral anticoagulants, or dual antiplatelet therapy after LAAC according to the studied regimen and instructions for use for each specific device and tailored to the bleeding risks of each patient.
12. TEE or cardiac computed tomography is recommended at 45 to 90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
13. Combined procedures with LAAC (e.g., structural interventions, pulmonary vein isolation) are not routinely recommended, as data are pending from ongoing randomized controlled trials.
“This consensus statement demonstrates the evolvement of LAAC treatment since our first statements that were issued in 2015 and 2016,” stated Jacqueline Saw, MD, FSCAI, chair of the writing group and an interventional cardiologist at Vancouver General Hospital and St Paul’s Hospital, Clinical Professor of Medicine at UBC, and Program Director of the VGH Interventional Cardiology Fellowship Program. “Since then, the results from several important clinical trials and registries, as well as other technological and clinical advancements have evolved and changed the way we look at operator requirements, patient selection, and shared decision making, which explains the need for this updated guidance.”
This statement has been developed according to the SCAI Publications Committee policies for writing group composition, disclosure, and management of relationships with industry, internal and external review, and organizational approval. The writing group has been organized to ensure diversity of perspectives and demographic characteristics, multistakeholder representation, and appropriate balance of relationships with industry. Relevant financial disclosures are available in the manuscript.
The American College for Cardiology and the Society of Cardiovascular Computed Tomography endorsed the statement.


 July 31, 2024
July 31, 2024 









