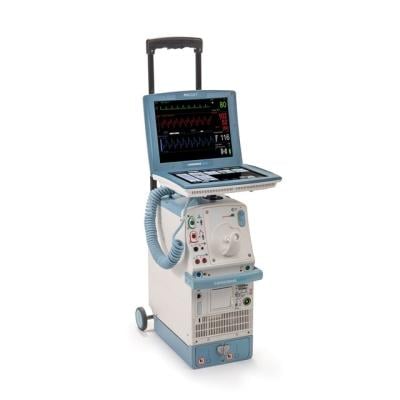
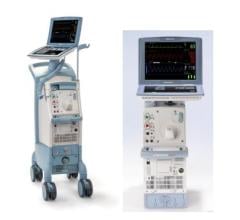
January 25, 2023 — According to a new release from the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary of Getinge, is recalling Cardiosave Hybrid IABPs and Rescue IABPs because a compromised intra-aortic balloon (a burst, leaking, or torn balloon) can cause blood to enter the IABP during therapy. Blood in the pump can cause risks such as an unexpected device shutdown, helium to be released into the patient’s blood, patient blood loss, potential blood cross-contamination between patients and/or a potential biohazard to the user and/or the service personnel.
The U.S. Food and Drug Administration (FDA) has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries, serious health consequences, or death.
Recalled Product
Product Name:
- Cardiosave Hybrid Intra-Aortic Balloon Pump (IABP) and Cardiosave Rescue Intra-Aortic Balloon Pump (IABP)
- Product Models: See Medical Device Recall Database entry
- Distribution Dates: March 6, 2012 to present
- Devices Recalled in the U.S.: 4,454
- Date Initiated by Firm: December 19, 2022
Device Use
The Cardiosave Hybrid Intra-Aortic Balloon Pump (IABP) and the Cardiosave Rescue IABP are electromechanical systems used to inflate and deflate intra-aortic balloons. These systems provide temporary support to the left ventricle through counter pulsation. Once the balloon is positioned in the aorta, the pump is set to work in synchrony with the electrocardiogram or arterial pressure waveform to make the balloon inflate and deflate at the right time during the cardiac cycle.
Cardiosave Intra-Aortic Balloon Pumps are indicated for acute coronary syndrome, cardiac and non-cardiac surgery, or complications of heart failure in adults. They are used in health care facilities.
Reason for Recall
Datascope, a subsidiary of Getinge, is recalling Cardiosave Hybrid IABPs and Rescue IABPs because a compromised intra-aortic balloon (a burst, leaking, or torn balloon) can cause blood to enter the IABP during therapy (a blood back event). Blood in the pump can cause the following issues:
- Unexpected pump shutdown if blood comes in contact with electrical components. A pump shutdown can lead to unstable blood flow (hemodynamic instability), organ damage and/or death, especially for people who are critically ill and most likely to receive therapy using these devices.
- If therapy continues, patients may have helium released into their blood. Helium gas bubbles (gas emboli) in the blood can damage organs (including the brain).
- Patient blood loss
- The user and/or subsequent maintenance or service personnel can be exposed to an unexpected biohazard should proper containment precautions not be taken.
- If the affected IABP is not evaluated before use with a new patient, that new patient may be exposed to cross-contaminated blood, which also exposes them to the potential for life-long bacterial and viral diseases such as hepatitis B, hepatitis C, and HIV.
Datascope/Getinge has reported 134 complaints about this issue, including 12 device shutdowns and 5 adverse events (4 serious injuries and 1 death).
Who May be Affected
- People who receive circulatory support using a CardioSave Hybrid or Rescue IABP
- Health care personnel providing care that includes the CardioSave Hybrid or Rescue IABP
- Users, and/or maintenance or service personnel providing service to the CardioSave Hybrid or Rescue IABPs
What to Do
On December 19, 2022, Datascope/Getinge sent customers an Urgent Medical Device Recall letter. The letter offered the following clinical guidelines and user actions.
Clinical Guidelines
- Do not bypass intra-aortic balloon (IAB) alarms and pay close attention to alarm notifications as that may help identify a perforated balloon earlier and prevent blood from traveling into the IABP. These include:
- Autofill Failure – Blood Suspected
- Autofill Failure
- Gas Gain in IAB Circuit
- Gas Loss in IAB Circuit
- IAB Catheter Restriction
- Periodically check the IAB catheter tubing for blood both throughout therapy and when the alarms occur. If any blood is noted or perforation is suspected, the following procedure must be performed immediately:
- Stop pumping by placing IABP console in Standby.
- Disconnect the catheter extender tubing from the IABP console to allow the balloon to deflate.
- Clamp extracorporeal tubing between white y-fitting and male connector.
- Place the patient in Trendelenburg as tolerated to guide any residual helium to travel away from the head vessels.
- Notify physician and prepare for IAB catheter removal.
- Consider IAB catheter replacement if the patient’s condition warrants.
- If blood is suspected of having entered the pump, take pump out of service. Have it evaluated before further use by Biomed/Technical Service to determine if replacement of contaminated components are necessary.
The letter’s guidance augments current clinical recommendations for how to manage patients if an intra-aortic balloon is damaged. It also notes that clinical care providers should maintain established patient and device management strategies regarding timing to IAB catheter removal.
Device User Actions
- Examine inventory immediately to identify any Cardiosave Hybrid and/or Rescue IABPs.
- If a blood back event is suspected, clinicians are to remove the Cardiosave from patient use, and report the event to appropriate biomedical engineering staff for inspection of the safety disk prior to next patient use.
- Ensure that all Cardiosave Intra-Aortic Balloon Pump users are aware of this notice and actions to perform.
- Distribute the above clinical guidance to users per institution policy.
- Complete and sign the MEDICAL DEVICE CORRECTION – RESPONSE form included with the letter to acknowledge receipt of notification. Return the completed form to Datascope/Getinge by e-mailing a scanned copy to [email protected] or by faxing the form to 1-877-690-5160.
- Distributors should forward the letter to any customers who received affected products.
Contact Information
Customers with questions about this recall should contact their Datascope/Getinge representative or call Datascope/Getinge Technical Support at 1-888-943-8872, options 4, 2, 1, Monday through Friday, between the hours of 8:00 a.m. and 6:00 p.m. (Eastern Time).
Related content:
Getinge Maquet/Datascope Intra-Aortic Balloon Pump (IABP) Shortage - Letter to Health Care Providers
FDA Class 1 Recall of Maquet Datascope IABPs Due to Fluid Seepage

 August 14, 2023
August 14, 2023