This is one of the images from the NIH photo gallery of COVID-19 images it has captured as part of its research on the virus. The full gallery can be found at https://www.flickr.com/photos/nihgov/albums/72157713108522106/with/49807561242/
In an effort to keep the imaging field updated on the latest information being released on coronavirus (COVID-19), the DAIC editorial team will be providing regular recaps of pertinent information.
Monday, May 4:
NIH Novel Coronavirus Photo Gallery
The National Intitues of Health (NIH) created a public photo gallery of the SARS-CoV-2 images it has captured from research on the novel coronavirus. It is available at https://www.flickr.com/photos/nihgov/albums/72157713108522106/with/49807561242/

NIAID Strategic Plan Details COVID-19 Research Priorities
Urgent public health measures are needed to control the spread of the novel coronavirus (SARS-CoV-2) and scientific research is needed to improve our understanding of the virus and how it causes disease, and to develop strategies to mitigate illness and death. A new strategic plan from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, details the institute’s plan for accelerating research to diagnose, prevent and treat COVID-19. It has four key priorities.
1. Improving fundamental knowledge of SARS-CoV-2 and COVID-19, including studies to characterize the virus and better understand how it causes infection and disease. This research includes natural history, transmission and surveillance studies to determine why some individuals experience mild symptoms of infection while others become critically ill. The role of asymptomatic individuals in viral spread and the potential seasonality of viral circulation also need to be explored, according to the report. Additionally, small and large animal models that can recapitulate COVID-19 disease seen in humans must be developed.
2. Development of rapid, accurate diagnostics and assays to identify and isolate COVID-19 cases and track the spread of the virus. Molecular assays can detect low levels of SARS-CoV-2 and differentiate it from other related viruses. Researchers will work to improve the speed and accuracy of these diagnostic assays to mitigate the spread of the disease during the current outbreak and any future ones. Additionally, new and improved serologic assays to detect antibodies to the virus must be developed to enhance surveillance efforts and identify individuals who may have resolved a previous COVID-19 infection.
3. Characterizing and testing potential treatments for COVID-19. These efforts will include identifying and evaluating drugs already approved for other conditions that could be repurposed to treat COVID-19 and testing novel broad-spectrum antivirals, such as remdesivir; virus-targeted antibody-based therapies; monoclonal antibodies; and host-directed strategies to target an individual’s immune response to the virus. To optimize findings during the pandemic, multiple clinical trials will be conducted in parallel among various patient populations, including hospitalized people and outpatients.
4. Develop safe and effective vaccines to protect individuals from infection and prevent future SARS-CoV-2 outbreaks. NIAID researchers and their collaborators are adapting vaccine candidates and approaches previously employed to address the related Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses and applied them to the current pandemic. For example, NIAID recently launched a Phase 1 clinical trial using a vaccine platform initially developed to target MERS. NIAID will use its broad clinical trial infrastructure to advance experimental vaccines through Phase 1 safety and dosing testing and simultaneously plan for advanced clinical testing of the most promising candidates. The institute will work with government partners to ensure that any safe and effective vaccine will be manufactured in sufficient quantities to allow expedient distribution to those at highest risk for infection.
Expert U.S. Panel Develops NIH Treatment Guidelines for COVID-19
A panel of U.S. physicians, statisticians and other experts has developed treatment guidelines for coronavirus for healthcare providers. It is are based on published and preliminary data and the clinical expertise of the panelists, many of whom are frontline clinicians caring for patients during the rapidly evolving pandemic. The guidelines are posted online at covid19treatmentguidelines.nih.gov and will be updated often as new data are published in peer-reviewed scientific literature and other authoritative information emerges.
The guidelines consider two broad categories of therapies currently in use by healthcare providers for COVID-19: antivirals, which may target the coronavirus directly, and host modifiers and immune-based therapies, which may influence the immune response to the virus or target the virus.
The panel’s conclusions about treating COVID-19 with various agents that fall into these two classes of therapies are distilled in summary recommendations. Subsequently, the document provides background information about each agent—such as clinical data about its use, ongoing clinical trials, and known interactions with other drugs—that forms the basis for the Recommendation. Tables briefly outline the same information.
NIH Study Validates Decontamination Methods for Re-using N95 Masks
N95 respirators can be decontaminated effectively and maintain functional integrity for up to three uses, according to National Institutes of Health (NIH) scientists. N95 respirators are designed for single-use and are worn by healthcare providers to reduce exposure to airborne infectious agents, including the virus that causes COVID-19. The study was conducted in a controlled laboratory setting, and the results were posted on a preprint server on today(link is external). The findings are not yet peer-reviewed, but are being shared to assist the public health response to COVID-19.
The study investigators are with NIH’s Rocky Mountain Laboratories (RML) in Hamilton, Montana, part of the National Institute of Allergy and Infectious Diseases (NIAID). With collaborators from the University of California, Los Angeles, they tested the decontamination of small sections of N95 filter fabric that had been exposed to SARS-CoV-2, the virus that causes COVID-19. Decontamination methods tested included:
• Vaporized hydrogen peroxide (VHP);
• 70-degree Celsius dry heat;
• Ultraviolet light; and
• 70 percent ethanol spray.
Wednesday, April 22:
U.S. COVID-19 Deaths Passes Annual Flu Deaths in Just One Month
The Centers for Disease Control and Prevention (CDC) reported 34,000 flu deaths during the 2018-19 flu season over the course of 7 months. The number of U.S. COVID-19 deaths rapidly rose between March and April, and are now at 45,525 U.S. deaths as of April 22, 2020.
Here are COVID-19 (SARS-CoV-2) stats from April 22, 2020:
• 2,593,129 worldwide diagnosed cases
• 827,038 diagnosed cases in the U.S.
• 45,525 U.S. deaths
• Mortality rate range for U.S cases between 2-7.5 percent, but are consistently between 6-10 percent in patients with various comorbitities
• The CDC reported April 16, 2020, 18.8% of all deaths occurring in the U.S. during the week ending April 11, 2020 (week 15) were due to pneumonia, influenza or COVID-19. This percentage is above the CDC epidemic threshold of 7% for week 15 and has been increasing sharply since the end of February.
Wednesday, April 15:
Rapid Drop in Heart Attacks and Stroke at Hospitals Concerns ACC
Since the start of COVID-19 containment efforts across the U.S. in March 2020, there has been a large drop in the normal number of heart attack and stroke cases showing up at hospitals across the country. This has raised concerns that patients are not seeking medical attention because of fears about going to the hospital during the epidemic. This prompted the American College of Cardiology (ACC) April 14 to begin a campaign targeting the public to urge people with what appear to be heart attack and stroke symptoms to seek medical help. The ACC is also offering new CardioSmart resources outline to help patients determine when and how to seek help.
ASE Sets Guidelines to Protect Echocardiographers During COVID-19
Echocardiographic services remains crucial during the COVID-19 outbreak. To minimizing risks to cardiac ultrasound staff, the American Society of Echocardiography (ASE) released a statement outlining recommendations to improve safety and reduce the potential for cross contamination. It addresses triaging and decision pathways for handling echocardiographic requests, as well as indications and recommended procedures to be followed for an echocardiographic assessment of cardiovascular function in suspected or confirmed COVID-19 cases. In addition, the document lists measures recommended to be used in the echo lab for prevention of spread of disease.
The statement says this requires judicious use of personal protective equipment (PPE), following best practices, pre-planning and exams and throughly sterilizing equipment after each exam.
Read the document at www.asecho.org/ase-statement-covid-19
VIDEO: Triage Considerations for Structural Heart Patients During COVID-19 From ACC and SCAI
In this video, Ehtisham Mahmud, M.D., FSCAI, president of the Society for Cardiovascular Angiography and Interventions (SCAI) and chief, Division of Cardiovascular Medicine at UC San Diego Medical Center, explains the new American College of Cardiology (ACC) and SCAI precaution guidelines for treating transcatheter aortic valve replacement (TAVR) patients in the cath lab during the COVID-19 pandemic.

SCAI President Ehtisham Mahmud explains new triage guidelines for structural heart patients during the COVID-19 pandemic.
Canadian Point-of-Care Ultrasound Guidelines Under COVID-19
The Canadian Society of Internal Medicine (CSIM) released recommendations for point-of-care ultrasound (POCUS) use during Coronavirus (COVID-19) pandemic, available at cjgim.ca/index.php/csim/article/view/438/1063.
Cardiology Vendor Baylis Medical to Manufacture Ventilators in Support of Canadian COVID-19 Response
Baylis Medical, a Canadian-based medical device company specializing in cardiology and spine, announced it is partnering with Ventilators for Canadians, a consortium of Canadian manufacturers, to manufacture ventilators for hospitals across Canada. This effort will aid the response to the novel coronavirus pandemic. The Government of Canada recently announced its support for Canadian manufacturing companies producing critical medical supplies, enabling Baylis to do its part to support the management of patients impacted by the COVID-19 virus. The company said it already has existing medical equipment manufacturing capabilities that made it well-positioned to provide these much-needed devices.
Clinical Features of Moderately to Critically Ill Patients with COVID-19 Who Die
In a retrospective case series in the New England Journal of Medicine (NEJM) of moderately to critically ill patients with confirmed COVID-19 from Wuhan, China, researchers assessed clinical features of who died and those who recovered. From mid-January to mid-February 2020, 799 patients were admitted to Tongji Hospital, which was constructed and designated as a hospital for moderately to critically ill COVID-19 patients. By February 28, 2020, 14% of patients had died, and 20% had recovered and been discharged. Median time from disease onset to death was 16 days.
Deceased patients, compared with patients who recovered, were older (age, 68 vs. 51), more likely to be male (73 vs. 55%), and more likely to have chronic hypertension and cardiovascular disease (48% vs. 14%). Deceased patients were more likely to have leukocytosis (50% vs. 4%) and lymphopenia (91% vs. 47%). Liver enzymes, creatinine, lactate dehydrogenase, troponin, N-terminal–pro–brain natriuretic peptide, d-dimer, and systemic inflammatory marker levels were much higher in deceased patients than in those who recovered. Acute respiratory distress syndrome (100%), sepsis (100%), acute cardiac injury (77%), heart failure (49%), acute kidney injury (25%), and encephalopathy (20%) were much more common in deceased patients.
Regardless of cardiovascular history, acute cardiac injury and heart failure were more common in deceased patients than in patients who recovered.
COVID-19 Hydroxychloroquine Treatment Brings Prolonged QT Arrhythmia Issues
Hydroxychloroquine and chloroquine are the new “medical miracles” being lauded by letters to the editor and anecdotal in-vitro/vivo studies, but the drugs carry risks such as cardiac ECG QT prolongation and subsequent arrhythmias, including torsade de pointes. Initial studies show significant reduction in COVID-19 viral “carriage” in patients receiving hydroxychloroquine and azithromycin, which has led to the off-label recommendation to use hydroxychloroquine for COVID-19. The drug is used to prevent or treat malaria infections caused by mosquitos and to treat auto-immune diseases such as lupus and rheumatoid arthritis when other medications have not worked or cannot be used. Read the article
New COVID-19 drug therapy guidance from the American Heart Association, the American College of Cardiology and the Heart Rhythm Society
The scientific community is learning more about the impact and interaction of cardiovascular diseases with SARS-CoV-2, including the impact of drug therapies being used and their negative cardiovascular impact. Together, the American Heart Association (AHA), the American College of Cardiology (ACC) and the Heart Rhythm Society (HRS) April 8 jointly published a new guidance, “Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 19) Treatment,” to detail critical cardiovascular considerations in the use of hydroxychloroquine and azithromycin for the treatment of COVID-19. Read the article
Monday, April 13:
COVID-19 Update—By the Numbers
At the time of reporting on Monday The New York Times reported at least 571,694 cases of novel coronavirus in the U.S. and more than 23,036 deaths. Bing’s COVID-19 Tracker reported at least 576,695 cases and 23,068 deaths. Globally, cases are over 1.9 million.
Electric Bands for Koreans who Break Quarantine
According to the Associated Press, in a controversial step, South Korea’s government says it will strap electronic wristbands on people who defy self-quarantine orders as it tightens monitoring to slow the spread of the new coronavirus. Senior Health Ministry official Yoon Tae-ho on Saturday acknowledged the privacy and civil liberty concerns surrounding the bands, which will be enforced through police and local administrative officials after two weeks of preparation and manufacturing. He said authorities need more effective monitoring tools because the number of people placed under self-quarantine has ballooned after the country began enforcing 14-day quarantines on all passengers arriving from abroad on April 1 amid worsening outbreaks in Europe and the United States.
Lee Beom-seok, an official from the Ministry of the Interior and Safety, admitted that the legal grounds for forcing people to wear the wristbands were “insufficient” and that police and local officials will offer consent forms for the devices while investigating those who were caught breaking quarantine. Under the country’s recently strengthened laws on infectious diseases, people can face up to a year in prison or fined as much as $8,200 for breaking quarantine orders. Lee said those who agree to wear the wristbands could be possibly considered for lighter punishment.
Italy Extends Lockdown
Italian Premier Giuseppe Conte has extended a nationwide lockdown and suspension of non-essential industrial production for another three weeks, through May 3, according to the Associated Press. Conte says in a nationally broadcast address that the sacrifices being made “were having results,” and that for this reason ‘’we cannot render vain the efforts taken. If we give in, we risk that all the positive results could be lost. It would be a great frustration for all, and we would have to start again, also with an increase in the number of dead.’’
The extension comes as the number of people in Italian hospitals and intensive care wards eases and the growth in the number of new cases and deaths narrows. Experts have said it will take a decrease in the number of cases to enter a so-called Phase II, allowing more freedom of movement to individuals but with precautions to guard against any new outbreaks.
New York’s Death Toll Hits 10,000
New York’s coronavirus death toll topped 10,000 only about a month after the state recorded its first fatality, Gov. Andrew Cuomo said Monday. The state tallied 671 new deaths on Sunday. It was the first time in a week the daily toll dipped below 700. Still, the governor noted people are still dying at a “horrific level of pain and grief and sorrow.” Hospitals are still getting 2,000 new patients a day, Cuomo said.
New York has now reported 10,056 deaths since early March, with more than half of them in the past week.
Wednesday, April 8:
FDA Approves ECMO to Treat COVID-19 Patients
 The U.S. Food and Drug Administration (FDA) has issued guidance to provide a policy to help expand the availability of devices used in extracorporeal membrane oxygenation (ECMO) therapy to address the novel coronavirus (COVID-19, SARS-CoV-2) public health emergency. ECMO systems are used for long-term respiratory/cardiopulmonary failure and provide assisted extracorporeal circulation and physiologic gas exchange of the patient’s blood for more than six hours.
The U.S. Food and Drug Administration (FDA) has issued guidance to provide a policy to help expand the availability of devices used in extracorporeal membrane oxygenation (ECMO) therapy to address the novel coronavirus (COVID-19, SARS-CoV-2) public health emergency. ECMO systems are used for long-term respiratory/cardiopulmonary failure and provide assisted extracorporeal circulation and physiologic gas exchange of the patient’s blood for more than six hours.
As a sudden acute respiratory syndrome, COVID-19 can trigger acute respiratory failure and/or acute cardiopulmonary failure. Under these conditions, the FDA states in the new policy extracorporeal oxygenation can now be used for greater than six hours as tool for treating patients. The FDA said it recognizes the importance and utility of increased availability of ECMO devices for patients during the COVID-19 emergency.
Image Gallery Showing Impact of the COVID-19 Pandemic
 DAIC and ITN created a photo gallery designed to show the impact of the novel coronavirus (COVID-19, SARS-CoV-2) on both healthcare and society world wide. The images are from staff members, submitted from readers on social media, and shared from press releases, hospitals and government public relations sources. This page will be updated regularly with new photos.
DAIC and ITN created a photo gallery designed to show the impact of the novel coronavirus (COVID-19, SARS-CoV-2) on both healthcare and society world wide. The images are from staff members, submitted from readers on social media, and shared from press releases, hospitals and government public relations sources. This page will be updated regularly with new photos.
Readers are welcome to submit their photos and caption information to [email protected].
Medtronic Shares its Designs to Accelerate Efforts to Increase Global Ventilator Production
Medtronic announced March 30 it is publicly sharing the design specifications for the Puritan Bennett 560 (PB 560) ventilator to enable other companies to evaluate options for rapid ventilator manufacturing to help doctors and patients dealing with COVID-19. This decision is consistent with the recent FDA Guidance and in accordance with the public health and medical response of governmental agencies globally. Medtronic.com/openventilator.
Introduced in 2010, the PB 560 is sold in 35 countries around the world. This ventilator’s ability to be used in a range of care settings, as well as its technology and design, make it a solid ventilation solution for manufacturers, inventors, start-ups, and academic institutions seeking to quickly ramp up ventilator design and production. PB 560 product and service manuals, design requirement documents, manufacturing documents, and schematics are now available at Medtronic.com/openventilator. The PB 560 design specifications are available today, software code and other information will follow shortly The PB 560 ventilator is a compact, lightweight, and portable ventilator that provides airway support for both adults and children. It can be used in clinical settings and at home and provides mobile respiratory support.
COVID-19 Update — By the Numbers
At the time of reporting on Wednesday The New York Times reported at least 418,185 cases of novel coronavirus in the U.S. and more than 14,257 deaths. Bing’s COVID-19 Tracker reported at least 427,118 cases and 14, 556 deaths. Globally, cases are over 1.5 million.
New York’s Grim COVID-19 Milestone
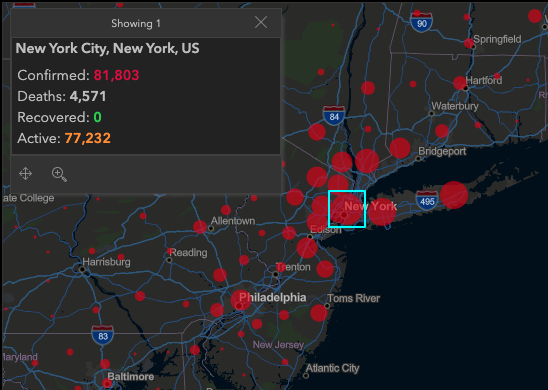 The Associated Press reports that New York City's death toll from the coronavirus rose past 4,000 on Tuesday, eclipsing the number killed at the World Trade Center on 9/11. This development came even as the crisis seemed to be easing or at least stabilizing, by some measures, in New York and parts of Europe, though health officials warned people at nearly every turn not to let their guard down. After 76 days, China finally lifted the lockdown on Wuhan, the city of 11 million where the outbreak began.
The Associated Press reports that New York City's death toll from the coronavirus rose past 4,000 on Tuesday, eclipsing the number killed at the World Trade Center on 9/11. This development came even as the crisis seemed to be easing or at least stabilizing, by some measures, in New York and parts of Europe, though health officials warned people at nearly every turn not to let their guard down. After 76 days, China finally lifted the lockdown on Wuhan, the city of 11 million where the outbreak began.
COVID-19's toll in New York City is now more than 1,000 deaths higher than that of the deadliest terror attack on U.S. soil, which killed 2,753 people in the city and 2,977 overall, when hijacked planes slammed into the twin towers, the Pentagon and a Pennsylvania field on Sept. 11, 2001. New York state recorded 731 new coronavirus deaths, its biggest one-day jump yet, for a statewide toll of nearly 5,500, Gov. Andrew Cuomo said. For more information
Map showing the statistics for New York April 8 are from the John Hopkins University Coronavirus Resource Center.
Florida Atlantic University Finds Simple Solution to Make Thousands of Face Shields for Baptist Health South Florida
 With major shortfalls of personal protective equipment (PPE) for healthcare workers around the world, a team from Florida Atlantic University’s College of Engineering and Computer Science and Institute for Sensing and Embedded Network Systems (I-SENSE), have identified a simple solution to rapidly produce protective face shields for Baptist Health South Florida, the largest healthcare organization in the region with 11 hospitals. The disposable face shield developed by the FAU team only requires clear polyester plastic, elastic fabric bands and a laser cutter. Unlike 3-D printed solutions, the process developed by FAU is simple and quick. Baptist Health South Florida has requested an initial order of 4,000 face shields, which the FAU team expects to have completed within a week and will be followed by another order of 4,000 face shields.
With major shortfalls of personal protective equipment (PPE) for healthcare workers around the world, a team from Florida Atlantic University’s College of Engineering and Computer Science and Institute for Sensing and Embedded Network Systems (I-SENSE), have identified a simple solution to rapidly produce protective face shields for Baptist Health South Florida, the largest healthcare organization in the region with 11 hospitals. The disposable face shield developed by the FAU team only requires clear polyester plastic, elastic fabric bands and a laser cutter. Unlike 3-D printed solutions, the process developed by FAU is simple and quick. Baptist Health South Florida has requested an initial order of 4,000 face shields, which the FAU team expects to have completed within a week and will be followed by another order of 4,000 face shields.
Leaders from Baptist Health South Florida approached FAU leadership to help them ramp up their acquisition of PPEs for their health care force, which includes approximately 23,000 employees, more than 4,000 physicians and more than 100 outpatient centers, urgent care facilities and physician practices spanning across Miami-Dade, Monroe, Broward and Palm Beach counties.
American Association of Critical-Care Nurses Says Lack of PPE Poses Clear and Present Danger to Nurses and the Nation
Without immediate action, limited supplies of personal protective equipment (PPE), ventilators and other lifesaving equipment will cause greater loss of life and increase the toll from COVID-19, warned the American Association of Critical-Care Nurses (AACN). The group represents more than half a million acute and critical care nurses, many of whom are caring for patients with COVID-19.
The AACN said to alleviate the strain on the healthcare system immediate action is needed:
• The federal government must immediately use its authority to enact the full Defense Production Act and order the rapid production and distribution of PPE
• Businesses should donate any excess PPE inventory to hospitals and other healthcare settings
• Manufacturers able to produce PPE should begin to do so now to help increase the inventory nationwide
• Individuals should heed the orders to shelter at home and practice social distancing to slow the spread of the virus and further protect themselves, their communities and the healthcare workforce
Tuesday, April 7:
COVID-19 Update—By the Numbers
At the time of reporting on Tuesday, The New York Times reported at least 380,000 cases of novel coronavirus in the U.S. and nearly 12,000 deaths. Bing’s COVID-19 Tracker reported at least 392,012 cases and 12,370 deaths. Globally, cases are over 1.4 million.
Coronavirus Research Index Reveals Countries Putting the most Effort in COVID-19 Research
Finbold.com has launched the Coronavirus Research Index (CRI) to identify countries that are putting in the most effort in finding ways to manage the COVID-19 disease. The index ranks the countries based on the number of active medical coronavirus studies that show which countries are actually executing the most research related to COVID-19 to understand the virus to find the effective means of managing the disease. According to the Index, China, the United States, and France are the top three countries leading in the number of active studies related to coronavirus.
The CRI shows that China has 60 active studies, with the United States having 49 ongoing studies. France has 26 active studies. Idas Keb, co-founder at Finbold, commented on the findings: “Interestingly, the index also reveals that while there is some correlation between countries that have the most COVID-19 cases and the number of medical studies, the majority of the countries are still far behind on coronavirus research. For example, Spain, which is second by the number of confirmed coronavirus cases, is not within the top 5 countries in the research index.”
Currently, the Finbold.com Coronavirus Research Index identifies 39 countries with ongoing studies on COVID-19. lcountries have confirmed cases of the novel coronavirus. You can find the research and try out the tool here: https://finbold.com/coronavirus-research-index/
European Commission Postponing MDR Due to COVID-19
Following the announcement by the European Commission to postpone the new Medical Devices Regulation (MDR) by one year in order to prioritize the fight against the coronavirus outbreak. Aliyah Farouk, medical devices analyst at GlobalData, a leading data and analytics company, offers her view: “The European Commission’s decision to postpone the application of the MDR is expected to disrupt approval and re-certification of devices, taking the pressure off many companies. The new MDR, set to come into action on 26 May 2020, required companies to undertake lengthy technical preparations needed for the market approval of their products. According to GlobalData estimates, there are over 200 coronavirus diagnostic products in the market with many more anticipated to be released in the coming months.
“Under the current Medical Device Directive (MDD) regulations, the in-house reuse of single-use devices is permitted through design modifications and cleaning/decontamination,” Farouk continued. “However, the MDR states single-use devices are to be professionally remanufactured. With the COVID-19 pandemic causing shortages in personal protective equipment (PPE) such as facemasks, many institutions have had to reuse equipment in order to meet the demand.
“The postponement therefore takes into account the challenges of the coronavirus pandemic and the need for increased availability of vital medical devices across Europe. As the outbreak has heightened demands for certain devices, postponing the MDR ensures market disruptions for critical devices is limited,” Farouk concluded.
For more information: www.globaldatacom
7Chairs Launches Telesupport Groups To Manage Stress and Anxiety
7Chairs, a technology company specializing in providing professionally facilitated online emotional support groups, is launching coronavirus topic-specific groups in order to ease the feelings of anxiety and stress people are feeling due to the coronavirus. These newly developed coronavirus-specific support groups will focus on topics such as coping with the fear, anxiety, and shock of coronavirus, isolation and loneliness as the result of quarantine or social distancing, parenting during coronavirus, unemployment and financial stressors, and grieving the loss of a loved one from coronavirus. All the groups are conducted by CHAT to allow anonymity and privacy.
These newly developed coronavirus-specific support groups will focus on topics such as coping with the fear, anxiety, and shock of coronavirus, isolation and loneliness as the result of quarantine or social distancing, parenting during coronavirus, unemployment and financial stressors, and grieving the loss of a loved one from coronavirus. All the groups are conducted by CHAT to allow anonymity and privacy.
To date, the 7Chairs platform has helped over 3,000 individuals find the emotional support they need by providing 450+ professionally facilitated topic-specific support groups. Each of 7Chairs' support groups consists of six participants and a professional facilitator. The 7Chairs Group Facilitators are all licensed Clinical Social Workers (LCSW or LICSW) who have experience in online group facilitation. The Corona-specific telesupport groups will be subsidized to the public.
With this unprecedented pandemic come increased levels of stress and anxiety that are often best addressed by professional treatment and support. Studies show that participating in online group support is extremely effective in reducing stress and anxiety. Additional benefits include feeling less lonely and more connected.
You can learn more about the program here: https://7chairs.co/corona-groups/
Friday, April 3:
Enforcement Policy for Face Masks and Respirators (Revised)
On April 2, 2020, the United States Food and Drug Administration (FDA) revised an immediately in effect guidance to provide a policy to help expand the availability of general use face masks for the general public and particulate filtering facepiece respirators (including N95 and KN95 respirators) for health care professionals during this pandemic. This guidance updates the previous version of the guidance published on March 25, 2020. This update provides recommendations regarding face shields, surgical masks, and alternatives, which may include KN95 respirators, when FDA-cleared or NIOSH-approved N95 respirators are not available. For more information: https://bit.ly/3dRyDU2
Best Practices for Nuclear Imaging During the Coronavirus (COVID-19) Pandemic
A new guidance document on best practices to maintain safety and minimize contamination in nuclear imaging labs from novel coronavirus (COVID-19, SARS-CoV-2) was created and released this week in partnership between the American Society of Nuclear Cardiology (ASNC) and the Society for Nuclear Medicine and Molecular Imaging (SNMMI). The "Guidance and Best Practices for Nuclear Cardiology Laboratories During the Coronavirus Disease 2019 (COVID-19) Pandemic: An Information Statement from ASNC and SNMMI," address principles for COVID-19 protection and offer specific recommendations for adapting nuclear cardiology practices at each step in a patient’s journey through the lab—for inpatients, outpatients and emergency department patients. You can view the full statement here: https://zenodo.org/record/3738020#.XodO2NNKine
The Industry Loses a Director of Radiology to COVID-19
The radiology world has lost a dedicated leader due to coronavirus (COVID-19). On March 29, Jeannie Danker, director of radiology at The Ohio State University Wexner Medical Center, died from suspected complications from the virus. She had worked at the center for more than 30 years. It was there that she met her husband, John, who tragically died after a struggle with Amyotrophic Lateral Sclerosis (ALS) just two months prior. “We will miss her friendship as well as her leadership and commitment to our institution, where she spent more than 30 years of her career,” the hospital said in a statement. “We extend our deepest sympathies to Jeannie’s family and those close to her.”
Healthcare Jobs Plummet
Despite overwhelming need for healthcare workers as the coronavirus continues to spread across the U.S., healthcare employment dropped by 43,000 in March, reversing a decade of job growth in the sector. For more information https://bit.ly/3aJwZSg
Virus Spread as of Friday morning
Globally, cases of COVID-19 surpassed 1 million on Thursday. As of Friday morning, The New York Times reported at least 244,000 cases in the U.S. and 6,200 deaths. At the time of reporting, Bing’s COVID-19 Tracker showed 245,974 cases in the U.S. and 6,126 deaths.
COVID-19 STEMI Registry Underway
The Society for Cardiovascular Angiography and Interventions (SCAI) and the Canadian Association of Interventional Cardiology (CAIC) announced the formation of the North American COVID-19 ST-Segment Elevation Myocardial Infarction Registry (NACMI). This will record data on COVID-19 patients who have STEMI. The purpose of the trial is to quickly gather information to understand the adverse cardiovascular effects being seen in COVID-19 patients.
Any COVID-19 positive patients or persons under investigation (PUI) with ST-segment elevation or new-onset left bundle branch block (LBBB) with a clinical correlate of myocardial ischemia (including chest pain, dyspnea, cardiac arrest, hemodynamic instability) will be in enrolled. The data will be compared to an age and gender-matched control population from the existing Midwest STEMI Consortium, which is a large (>15,000), prospective multi-center registry of consecutive STEMI patients. Interested sites can designate a local principal investigator and contact SCAI PI Tim Henry, M.D., at [email protected]. Read more on this study.
VIDEO: Telemedicine in Cardiology and Medical Imaging During COVID-19 — Interview with Regina Druz, M.D.
Regina Druz, M.D., FASNC, a member of the American Society of Nuclear Cardiology (ASNC) Board of Directors, chairwomen of the American College of Cardiology (ACC) Healthcare Innovation Section, and a cardiologist at Integrative Cardiology Center of Long Island, N.Y., explains the rapid expansion of telemedicine with the U.S. spread of novel coronavirus. Watch the video: https://bit.ly/39I2nPY
FCC Adopts $200 Million for COVID-19 Telehealth Program
The Federal Communications Commission (FCC) voted to adopt a $200 million telehealth program to support healthcare providers responding to the ongoing coronavirus pandemic. Congress appropriated the funds as part of the CARES Act last week. Through the COVID-19 Telehealth Program, the FCC will help healthcare providers purchase telecommunications, broadband connectivity, and devices necessary for providing telehealth services. Funding applications from healthcare providers will be processed on a rolling basis. The FCC also adopted final rules to stand up a Connected Care Pilot Program. This separate three-year Pilot Program will provide up to $100 million of support from the Universal Service Fund (USF) to help defray health care providers’ costs of providing connected care services and to help assess how the USF can be used in the long-term to support telehealth.
FDA Warns to Beware of Fraudulent Coronavirus Tests, Vaccines and Treatments
The U.S. Food and Drug Administration (FDA) released a warning this past week that there has been a flood of questionable products that claim to help diagnose, treat, cure, and even prevent COVID-19. Because COVID-19 has never been seen in humans before, there are currently no vaccines to prevent or drugs to treat COVID-19 approved by the FDA. The agency is working with vaccine and drug manufacturers to develop new vaccines for and find drugs to treat COVID-19 as quickly as possible. Meanwhile, some people and companies are trying to profit from this pandemic by selling unproven and illegally marketed products that make false claims, such as being effective against the coronavirus.
The FDA has also seen unauthorized fraudulent test kits for COVID-19 being sold online. Currently, the only way to be tested for COVID-19 is to talk to a healthcare provider. The FDA has not authorized any test that is available to purchase for testing yourself at home for COVID-19.
FDA Townhall Meeting on COVID-19 Diagnostic Tests
On Wednesday, April 8, 2020, from 12:15-1:15 pm Eastern Time, the U.S. Food and Drug Administration (FDA) will host a virtual Town Hall for clinical laboratories and commercial manufacturers that are developing or have developed diagnostic tests for SAR-CoV-2. During this Town Hall, the FDA will help answer technical questions about the development and validation of tests for SARS-CoV-2.
FDA Hosts Webinar on Enforcement Policy for Personal Protective Equipment (PPE) During COVID-19
The U.S. Food and Drug Administration (FDA) will host a webinar to share information and answer questions on the recently issued, immediately in effect guidance documents for its enforcement policy for face masks and respirators during the coronavirus public health emergency. The webinar is Monday, April 6, 2020, between 3-4 p.m. EST. No registration required.
View webinar details for call-in information.
Societies Create a COVID-19 Resource Pages for Cardiology
Several cardiology societies have created novel coronavirus resource pages with information and videos on the cardiovascular impact of COVID-19 and firsthand accounts on treating patients with the virus.
List of links to society pages.
Healthcare Societies Issue Urgent Call for Federal Action to Address COVID-19 Medical Equipment Shortages
A group of 13 U.S. medical societies sent an open letter to Congress appealing to them to do what is needed to ensure the supply of critically needed medical equipment in the fight against novel coronavirus (COVID-19, SARS-CoV-2). "We stand united in voicing our concern over the critical shortages of medical equipment, including ventilators, test kits and all types of personal protective equipment (PPE) such as masks, face shields and gowns, to adequately address the COVID-19 public health crisis," the leaders of the societies sated in the letter.
The societies included:
• American Heart Association (AHA)
• American College of Cardiology (ACC)
• Society for Cardiovascular Angiography and Interventions (SCAI)
• Association of Black Cardiologists
• Heart Failure Society of America
• Heart Rhythm Society (HRS)
• American Academy of Neurology
• American Academy of Pediatrics
• American Association for Respiratory Care
• American College of Emergency Physicians
• National Association of EMS Physicians
• National Registry of Emergency Medical Technicians
• Society of Diagnostic Medical Sonography
Lessons from COVID-19 Hotspots Podcast from the NEJM
The New England Journal of Medicine (NEJM) conducted a video interview April 1, in which the editors Eric J. Rubin, Lindsey R. Baden, and Stephen Morrissey draw lessons from the early COVID-19 outbreak in Seattle and the growing crisis in New York City.
Listen in and find more information.
Amid Severe Blood Shortage FDA Changes Donor Eligibility
The FDA announced it is revising recommendations in several guidances regarding blood donor eligibility. These changes are based on recently completed studies and epidemiological data, leading the FDA to conclude that the polices could be modified without compromising the safety of the blood supply. The changes being announced to three guidances (HIV, malaria and CJD/vCJD) are for immediate implementation, and are expected to remain in place after the COVID-19 pandemic ends, with any appropriate changes based on comments we receive and our experience implementing the guidances. Additionally, the FDA is publishing a fourth guidance providing notice of alternatives to certain requirements regarding blood donor eligibility for the duration of the COVID-19 pandemic.
The guidances are available on FDA’s web site.
New York Paramedics Call on Gov. Cuomo to Use Paramedics in Hospitals
In response to New York Gov. Cuomo’s request for suggestions to increase the capacity of dealing with COVID-19 cases, New York paramedic Robbie MacCue, co-founder of the EMS Leadership Academy, issued a letter urging New York state and other government officials to utilize trained paramedics who are, under normal circumstances, excluded from working in New York State hospital Emergency Departments or other acute care settings.
McCue is also asking the governor to ensure EMS systems have access to PPE stockpiles that may be reserved exclusively for hospitals, and that they use inclusive language when talking about healthcare providers, public safety, and first responders. Often in public speeches there is mention of doctors and nurses, or firemen and police, but there is little to no mention of EMS providers or paramedics and EMTs, despite their being the backbone of the most rapid responses across the state amid this crisis.
CMS issues New COVID-19 Guidance for Nursing Homes
The Centers for Medicare & Medicaid Services (CMS) issued new Guidance for Infection Control and Prevention of Coronavirus Disease 2019(COVID-19) in Nursing Homes.






 September 24, 2025
September 24, 2025