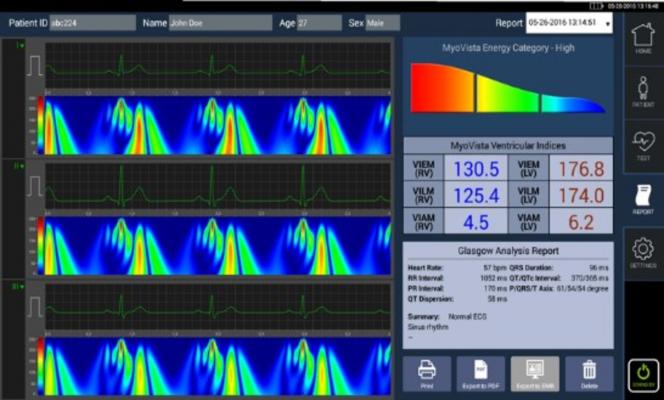
Just as a Doppler radar color image shows the energy of a storm, MyoVista provides physicians a detailed visual image of the energy distribution during the cardiac cycle.
August 22, 2017 — HeartSciences announced the European launch of the MyoVista high sensitivity electrocardiograph (hsECG) Testing Device, developed in response to the global unmet need for effective, low-cost, front-line screening of cardiac disease in both symptomatic and asymptomatic patients. MyoVista measures the heart's energy during each heartbeat using a type of advanced signal processing known as Continuous Wavelet Transform (CWT).
Advanced signal processing has revolutionized early detection and predictive accuracy in other applications like weather forecasting. Just as a Doppler radar color image shows the energy of a storm, MyoVista provides physicians a detailed visual image of the energy distribution during the cardiac cycle.
Electrical, structural and coronary artery disease (CAD) comprise the three main categories of heart disease, but existing ECG technology, which functions essentially in the same way as when first introduced in 1903, has limited effectiveness in detecting structural and CAD. Even though it is the front-line screening tool used by physicians to detect heart disease, conventional resting ECG technology detects CAD less than 50 percent of the time, which leaves a large number of patients who have heart disease undiagnosed. As a result, current healthcare guidance does not recommend use of ECG testing on asymptomatic patients.
"Currently, there's a significant diagnostic gap in detecting heart disease early, resulting in a burden on both patients and healthcare systems," said Andrew Simpson, chairman, HeartSciences. "We believe MyoVista hsECG could play an important role in achieving the preventative treatment ambitions of many healthcare systems as well as help reduce unnecessary healthcare expenditures."
The application of advanced signal processing technology can assist in closing the diagnostic gap by providing a low-cost, front-line tool that can assist in the early detection of cardiac dysfunction. During the validation trial, MyoVista hsECG technology detected cardiac dysfunction in the resting (diastolic) phase of the cardiac cycle with 88 percent sensitivity and 87 percent specificity. The 200-patient trial assessed the presence of left ventricular diastolic dysfunction.
"Innovation is needed to advance front-line testing of patients for heart disease," said Partho P. Sengupta, M.D., chief of cardiology and chair of cardiac innovation at West Virginia University, and lead investigator on a MyoVista clinical trial. "High sensitivity ECG technology holds significant promise for improving the detection of heart disease."
MyoVista hsECG uses the same 12-lead, at-rest testing protocol as traditional ECG devices on the market today to help facilitate easy adoption for clinical staff. The difference is that MyoVista is a single test that provides healthcare practitioners with unique informatics, as well as conventional 12-lead resting ECG tracings and conventional ECG interpretive analysis. In combination, these assist identification of cardiac dysfunction related to CAD and structural disease, as well as identifying arrhythmias.
MyoVista hsECG is available across the European Union, where it received the CE Mark approval for commercial sale. The company is also in the process of expanding distribution to Australia, the Middle East, Latin America, Asia-Pacific and Canada. The device is not currently available for sale or use in the United States. HeartSciences expects to seek U.S. Food and Drug Administration clearance for MyoVista in 2018.
For more information: www.heartsciences.com


 November 12, 2025
November 12, 2025