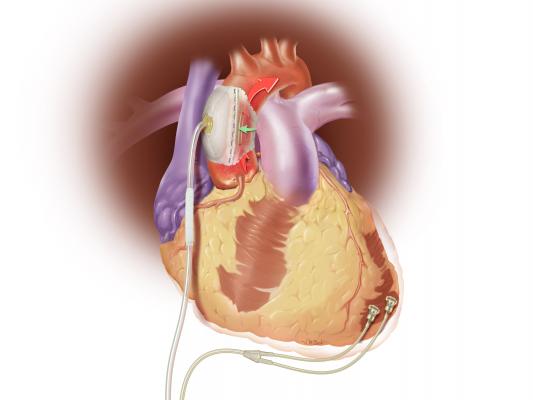
August 18, 2015 — Sunshine Heart Inc. announced an update in late July on its COUNTER HF U.S. pivotal study for the C-Pulse Heart Assist System. COUNTER HF is a prospective, randomized, multi-center, controlled study evaluating the safety and efficacy of the C-Pulse system for the treatment of New York Heart Association (NYHA) Class III and ambulatory Class IV heart failure. The study was temporarily paused this past March after the company notified the U.S. Food and Drug Administration (FDA) of four deaths in the treatment arm of the study. The deaths were adjudicated as not device- or therapy-related, and as previously announced on May 26th, the FDA approved resumption of patient enrollment in the study.
Immediately following the FDA's decision to approve enrollment to continue the COUNTER HF's study, Sunshine Heart distributed material to all sites with the necessary documentation in order to achieve site Investigational Review Board (IRB) approvals. Currently, 12 sites have been reactivated, which is approximately half of all previously activated sites. Sunshine Heart expects the majority of sites to be reactivated by the end of August.
In addition, the company announced the enrollment of its first two patients since the resumption of the COUNTER HF study. One of these patients was already reviewed by Sunshine Heart's newly formed Physician Subject Selection Committee. This led to the first implant being scheduled for the morning of July 23.
The C-Pulse System is an investigational device in the United States and Canada that utilizes the scientific principles of intra-aortic balloon counter-pulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants.
For more information: www.sunshineheart.com


 February 03, 2026
February 03, 2026 









