October 13, 2014 — After reviewing updated data and analysis for the Boston Scientific Watchman left atrial appendage (LAA) closure device, the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee voted in favor of the device. By a vote of 6 to 5 (with 1 abstention) the panel concluded the benefits of the Watchman device outweigh the potential risks.
Furthermore, the panel voted there was reasonable assurance the device is safe (12 yes to 0 no). On the question of reasonable assurance of effectiveness, the panel vote was unfavorable (6 yes to 7 no). The panel provided substantial input and guidance related to the proposed indications for use and target patient population. There was widespread agreement among the panel members that the device provides a much needed alternative to long-term anticoagulation for some patients. While not bound by this vote, the FDA takes Advisory Panel comments and recommendations into account when reviewing the Watchman device application. The company is committed to working with the FDA to address the panel's comments.
"There is a strong clinical need for a proven device alternative to long-term warfarin therapy for my high stroke risk patients with non-valvular atrial fibrillation," said Vivek Reddy, M.D., director of the Cardiac Arrhythmia Service at Mount Sinai Medical Center and co-principal investigator of the PROTECT AF and PREVAIL studies. "I'm encouraged that the panel recognized the importance of having the Watchman device as an option for appropriate patients."
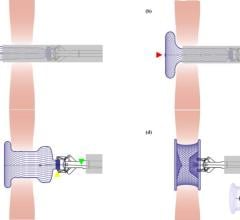
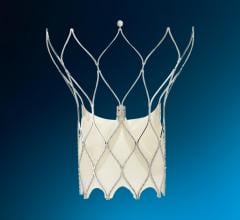
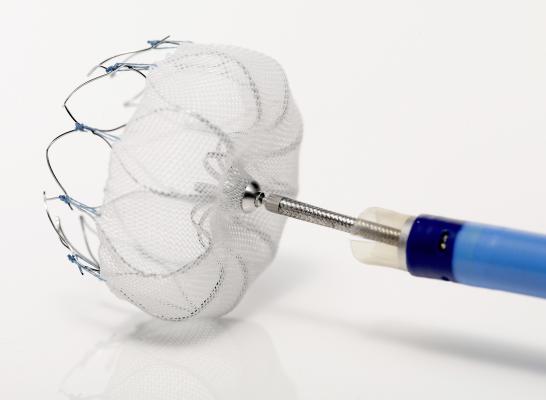
The Watchman device is a catheter-delivered heart implant designed to close the LAA in order to prevent the migration of blood clots from the LAA, and thus reduce the incidence of stroke and systemic embolism for higher risk patients with non-valvular atrial fibrillation (AF).
The committee's positive vote followed a review of the most recent clinical data and analysis from two randomized control trials, PROTECT AF and PREVAIL, as well as from the CAP (Continued Access Protocol) and CAP2 registries. The Watchman device is the most studied LAA device and the only one with long-term clinical data from more than 2,400 patients and nearly 6,000 patient-years of follow-up in clinical studies. The Watchman device was approved for sale in Europe in 2005 and is currently approved in more than 70 countries across the globe. In the United States, the Watchman device is an investigational device, limited to investigational use and not available for sale.
For more information: www.bostonscientific.com


 June 20, 2024
June 20, 2024