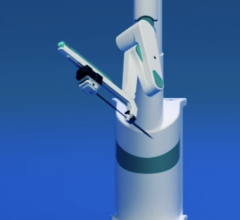
July 31, 2014 — As attention increases on the effects of radiation exposure among interventional cardiologists[1], Corindus Vascular Robotics announced the recent installations of its CorPath System at five facilities: UH Case Medical Center, Cleveland, Ohio; Virginia Commonwealth University Medical Center, Richmond, Va.; Miami Cardiac and Vascular Institute, Miami; the second system in Michigan; and first system in North Carolina. In addition, Corindus announced that customers used the CorPath System in a record number of cases in the quarter ending June 2014, the third straight quarter of record-setting utilization. The consistent increase in utilization demonstrates that customers continue to make CorPath the standard of care for PCI.
Corindus’ CorPath System is the first U.S. Food and Drug Administration (FDA)-cleared medical device to bring robotic-assisted precision and accuracy to coronary angioplasty procedures. During a CorPath Robotic Angioplasty procedure, the interventional cardiologist sits in the radiation shielded interventional cockpit, and advances stents and guidewires with millimeter-by-millimeter precision. Robotic angioplasty may improve stent placement, which can reduce repeat procedures. The CorPath System also reduces physician radiation exposure during procedures by 95 percent[2].
“We use the CorPath System on a daily basis at our facility. We even use the CorPath System as a tool with appropriate complex PCI cases, and continue to see and feel the benefits of robotic precision, along with radiation protection for the physician,” said Daniel Simon, M.D., director, Harrington Heart & Vascular Institute, UH Case Medical Center.
“The threat of radiation exposure and musculoskeletal disorders for interventional cardiologists is poised to become a pivotal industry issue as emerging studies continue to point to a real need for protecting clinicians in the cath lab,” said David Handler, President and CEO of Corindus Vascular Robotics. “We continue to meet with hospital executives who understand the potential cost and risk that radiation poses to their staff, and who see the CorPath System as a way to not only benefit patients, but their physicians as well.”
Corindus has seen the number of customer installs grow across the country, and several customers have recently added second systems to their facility to provide Robotic Angioplasty for more patients and physicians.
Corindus recently exhibited at the Society for Cardiovascular Angiography and Interventions (SCAI) 2014 annual scientific session, where more than 60 physicians attended a breakfast symposium sponsored by Corindus. Five current users spoke about their experience with CorPath and the benefits they derive from the implementation of robotic angioplasty.
For more information, visit www.corindus.com.
References:
1. Wood, Shelley. “Climbing Head and Neck Tumor Count in Interventional Cardiologists Prompts Calls for More Study,” MedScape.com, 23 April 2014, http://www.medscape.com/viewarticle/824022
2. Weisz, G. et al. Safety and Feasibility of Robotic Percutaneous Coronary Intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol. 2013;61(15):1596-1600.


 January 27, 2026
January 27, 2026