March 10, 2014 — ImaCor Inc., the developer of hemodynamic transesophageal echocardiography (hTEE) technology, announced four academic hTEE presentations. Physicians are publicly recognizing hTEE as a tool for managing complex ICU patients. Nick Fletcher, MBBS presented hTEE during the Heart Failure: State of the Art and Future Perspectives 5-day European Association of Cardio-Thoracic Surgery (EACTS) advanced module in Windsor, UK; Serban Bubenek, M.D., Ph.D described hTEE as the Advanced Hemodynamic Management solution at the Autumn Meeting of the European Society of Anesthesiology (ESA) in Timisoara, Romania; and Margarita Camacho, M.D. and Claudia Gidea, M.D. share hTEE case findings at the Tufts 6th Annual New England Heart Failure and Transplantation Network Conference in Waltham, Mass.
This follows an Oct. 8, 2013 session on hTEE Advanced Hemodynamic Management that was part of the scientific program at the 27th Annual Meeting of EACTS in Vienna, Austria. Speakers included A. Pieter Kappetein, M.D., Ph.D, Matthias Loebe M.D., Ph.D, and Simon Maltais M.D., Ph.D. During the 90-minute session, three videos of live hTEE case demonstrations were also presented from Vanderbilt University Medical Center, Nashville, Barnes-Jewish Hospital, St. Louis and Thomas Jefferson University Medical Center, Philadelphia.
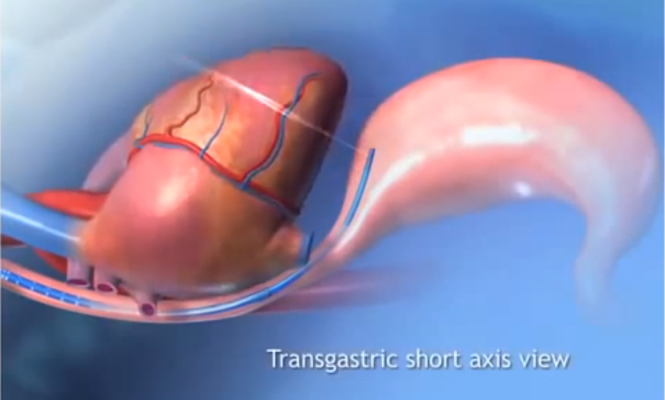
hTEE is enabled through a miniature, disposable probe that can be placed in a patient for up to 72 hours to directly observe cardiac filling and function over time. It is easy-to-use and purpose-built for the ICU staff. With the growing population of older, sicker patients with multiple comorbidities, physicians recognize the benefits of hTEE’s continuous hemodynamic management to guide therapy.
For more information: www.imacorinc.com


 June 13, 2024
June 13, 2024