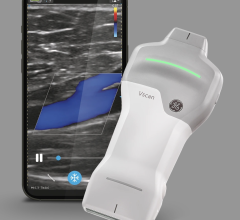
June 30, 2022 — Clarius Mobile Health, a leading provider of high-definition wireless ultrasound systems, and ImaCor Inc, the hemodynamic ultrasound company, today announced a partnership that enables the availability of the world’s first handheld transesophageal echocardiography (TEE) system designed to manage and guide care for the most critically ill patients in the Intensive Care Unit (ICU). The Zura Handheld Hemodynamic Ultrasound system powered by Clarius provides an instant, clear window to directly visualize preload and contractility over time, having received clearance by the U.S. Food and Drug Administration to help clinicians make the right decisions, at the right time.
“A high-risk patient’s hemodynamic status changes dramatically in a matter of minutes, often while clinicians are waiting for test results they submitted 15 minutes ago,” says ImaCor’s Founder, Scott Roth, MD. “The Zura Handheld provides instant and accurate information to guide patient management and it’s proven to save lives. More than 20,000 patients have already benefitted from our cart-based Zura system. We’re excited to make this life-saving device now highly accessible to more high acuity care teams with the introduction of our handheld system, which is easier to bring to the bedside, can be used to monitor multiple patients in real-time, and takes up no floor space. We chose to partner with Clarius for our handheld solution, because it delivers the highest image quality for the best patient care.”
According to Dr. Roth, hospitals will be able to purchase multiple Zura handheld systems for the cost of one cart-based system. The Zura Handheld Hemodynamic Ultrasound system is the companion device to the ClariTEE transesophageal echo probe. Now powered by Clarius, they comprise the first and only advanced handheld hemodynamic management platform for real-time decision making to care for the most at-risk patients. Pocket-sized, the 22-ounce handheld is a key advantage in the ICU where space is limited. The Zura Handheld operates with the Clarius Ultrasound App, which connects wirelessly to Apple and Android smart devices.
“Hemodynamic Ultrasound has played a vital role in saving lives in our most critically ill patient population,” commented Dr. Dennis Ashley, Director of Trauma and Adult Critical Care at Atrium Health Navicent. “Being the first clinical team to use the new Zura Handheld aligns with our focus on bringing the latest and most innovative care options to our patients. Portability means a member of our team can have a Zura Handheld with them, ready to use and available to identify the root cause of hemodynamic compromise and successfully guide patient management right at the bedside.”
“Our collaboration with ImaCor is a powerful example of how Clarius is broadening access to medical imaging and helping clinicians to provide better specialized patient care,” said Clarius Founder Laurent Pelissier. “We have intentionally designed our app-based ultrasound system to push the boundaries of medical imaging. It’s an honour to work with Dr. Roth and his team to make his vision for better acute care a reality, improving patient care and saving lives.”
For more information: www.clarius.com, or www.imacorinc.com.
Related ultrasound content:


 January 28, 2026
January 28, 2026