February 18, 2014 — Corindus Vascular Robotics is a developer of precision vascular robotics that present on robotics in the future of cath lab at the Cardiovascular Research Technologies (CRT) conference, Feb. 22–25, 2014 in Washington, D.C. Additionally, cases submitted by users of its CorPath Vascular Robotics System have been accepted for presentation at the conference.
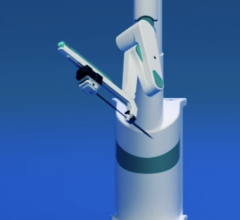
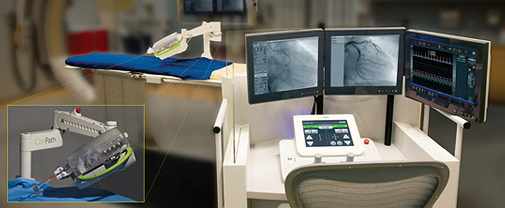
The CorPath System is an U.S. Food and Drug Administration (FDA)-cleared technology that enables precise, robotic-assisted angioplasties to treat coronary artery disease. Dr. Giora Weisz, chairman of cardiology at Shaare Zedek Medical Center in Israel, will discuss this technology in the “Cath Lab of the Future” and cases accepted to CRT will discuss current clinical use of CorPath.
Puneet Sharma, M.D., interventional cardiologist at Sanford Clinic in Aberdeen, S.D., had a case selected as an Interesting Case for the conference. Sharma’s case submission details the first robotic-assisted stent placement in acute heart-attack within the 90 minute door to balloon guideline. Sharma used CorPath to complete a procedure in 68 minutes of the patient’s arrival.
The CorPath System enables precisely controlled, robotic-assisted angioplasties while the physician is seated in a lead-lined interventional cockpit protected from radiation exposure. CorPath enables the cardiologist to advance stents and guidewires millimeter-by-millimeter via a joystick.
The Corpath system can be seen throughout the CRT conference in the following settings:
- Weisz's presentation, Feb. 25 at 2:10 p.m. in Palladian Room during the Cardiovascular Innovations session.
- Sharma’s submission will be presented online at CRTOnline.org.
- Corindus Vascular Robotics will be at booth #102 with a demo of the CorPath.
For more information: www.corindus.com


 January 27, 2026
January 27, 2026