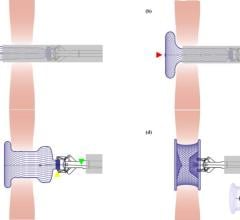
February 13, 2013 — St. Jude Medical has issued a Class I recall for its Amplatzer TorqVue FX Delivery System used in transcatheter closure of atrial septal defects (ASDs). The company said in a small number of cases, the distal end of the core wire of the TorqVue FX system can fracture, which has the potential to cause serious adverse health consequences, including death.
The FDA announced the recall today, but the vendor originally announced it Jan. 17, 2013 in an “urgent” recall notice sent to its customers. The device is used to assist the attachment, loading, delivery and deployment of Amplatzer occluder devices.
The recall includes product manufactured between Aug. 24, 2012 to Sept. 24, 2012 and distributed Oct.1, 2012 – Jan. 9, 2013. The affected model/catalog/lot numbers include: 9-ITVFX06F45/60, 9-ITVFX07F45/60, 9-ITVFX007F45/80, 9-ITVFX08F45/60, 9-ITVFX08F45/80, 9-ITVFX09F45/80, 9-ITVFX10F45/80, 9-ITVFX12F45/80, 9-ITVFX13F45/80.
For more information, customers with questions can contact the company at 651.756.6526.



 June 20, 2024
June 20, 2024