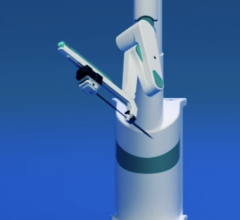
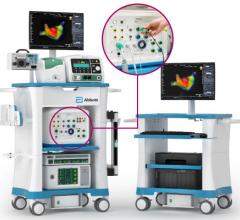
August 23, 2012 – Philips Healthcare and Corindus Vascular Robotics announced a distribution agreement for Corindus’ CorPath 200 System. The system was recently cleared by the U.S. Food and Drug Administration (FDA) and is the world's first robotic-assisted system for percutaneous coronary intervention (PCI) of obstructed coronary arteries.
The financial details of the exclusive distribution agreement were not disclosed. Philips owns a minority share in Corindus Vascular Robotics.
The CorPath 200 can be integrated with all major X-ray fluoroscopy systems, including Philips’ Allura X-ray equipment. The robotic-assisted system is a compact and cost-efficient system that can be used with standard stents, catheters and guidewires. The distribution agreement enables both companies to sell this unique robotic-assisted system in the United States, addressing an immediate need of patients, interventional cardiologists and staff. This agreement is the next step in the alliance between Philips and Corindus, which was announced in 2011.
“Robotic-assisted interventional technologies have great potential to further improve current image-guided minimally invasive procedures, which is a fast growing market with, for example, more than 1 million PCI procedures estimated to be performed in the United States in 2012,” said Gene Saragnese, CEO of imaging systems at Philips Healthcare. “By teaming up with partners with complementary and unique strengths, such as Corindus Vascular Robotics, we aim to shape the future of healthcare by delivering innovative solutions that enhance clinical capabilities, improve patient outcomes and reduce healthcare costs.”
X-ray fluoroscopy is generally accepted as the most suitable imaging technology for accurately visualizing the progress of the catheter and other interventional tools during the minimally invasive treatment of cardiac conditions. However, since interventional cardiologists perform many PCI procedures, over time clinical data has demonstrated that this can lead to health and orthopedic problems.
The CorPath 200 system has been designed for the robotic-assisted placement of the coronary guidewires and stent/balloon catheters used in PCI procedures. It is operated by the interventional cardiologist from a radiation-shielded, interventional cockpit. Additionally, the seated position in front of monitors may provide enhanced measurement and view of the angiography screen, while reducing fatigue and head, neck and back strain.
“At Northeast Georgia Medical Center, our group performs more than 2,000 procedures a year,” said Jeffrey Marshall, M.D., of the Northeast Georgia Heart Center. “Some of them lasting more than four hours – standing hunched over a bed and wearing a heavy lead apron. Robotic-assisted PCI allows us to perform the procedure shielded from radiation while precisely controlling the coronary guidewires and stent/balloon catheters. The CorPath 200 system may enhance precision and control of stent and guidewire positioning, which ultimately can potentially enhance patient care.”
The CorPath PRECISE Trial, a prospective, single-arm, multicenter study, which enrolled 164 patients and was submitted to FDA as part of system’s approval process, demonstrated robotic-assisted PCI is safe and feasible for patients. It also showed it can also significantly reduce radiation exposure for the interventional cardiologist.
“We are excited to have a strong partner in Philips, who has such an established reputation and presence in interventional cardiology,” said David Handler, president and CEO of Corindus. “Philips’ U.S. sales force has already been introduced to the CorPath 200 System, and U.S. commercialization is underway. Additionally, Corindus Vascular Robotics is also bolstering our U.S. sales force to provide clinical support to interventional cardiologists performing robotic-assisted PCI.”
For more information: www.philips.com/newscenter, www.corindus.com


 January 27, 2026
January 27, 2026