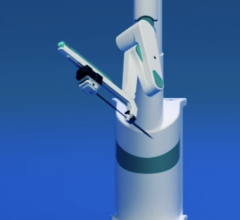
June 5, 2012 — Hansen Medical Inc. received 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for its Magellan Robotic System, the first robotic navigation system to more precisely guide peripheral artery disease (PAD) interventions in the cath lab. The approval includes the catheter and accessories for the system. The company will commence commercialization at selected centers in the United States immediately, with a full launch expected later in the year. The product will be presented at the 66th Vascular Annual Meeting of the Society for Vascular Surgery at National Harbor in Maryland from June 7-9.
The Magellan Robotic System is intended to facilitate navigation to anatomical targets in the peripheral vasculature and subsequently provide a conduit for manual placement of therapeutic devices. The system has the potential to provide vascular surgeons and other interventionalists the ability to perform fast and predictable procedures, while allowing the physician to be seated comfortably away from the radiation source, which may reduce radiation exposure and physician fatigue.
“Today’s announcement marks one of the most significant milestones in the company’s history,” said Hansen Medical President and CEO Bruce Barclay. “Not only does the Magellan System have the potential to be a significant growth driver for Hansen, it also represents a fundamental step forward in the transformation of vascular intervention using intravascular robotics.”
The global vascular market is large and expanding rapidly, driven by an aging population, the prevalence of diabetes and obesity, and an increase in disease awareness. Of the more than 3 million vascular procedures done worldwide each year, approximately one-third to one-half of them could be addressed using the Magellan Robotic System.
“The Magellan Robotic System is a significant technological advancement that may offer important clinical benefits for physicians performing peripheral interventions," said Alan Lumsden, M.D., chair of Hansen Medical’s U.S. Scientific Advisory Board, and chair of the department of cardiovascular surgery and medical director of Methodist DeBakey Heart and Vascular Center at The Methodist Hospital, Houston. "The system provides physicians with independent robotic control of both catheter tips to navigate efficiently through a variety of anatomies and lesions. Our in vitro and in vivo animal studies indicate that using this platform has the potential to increase efficiencies in the interventional lab by shortening procedure times and allowing more predictable interventions. Ultimately, we believe this system may facilitate alternative patient treatment options by enabling robotic endovascular interventions.”
The Magellan offers a new way to perform peripheral vascular interventions that has the potential to deliver revolutionary lesion access, precise distal tip control, solid catheter stability and consistent procedural efficiency.
“Since the Magellan Robotic System was designed specifically for vascular interventions, it offers excellent catheter stability and precision during the delivery and placement of a variety of therapeutic devices in different anatomic conditions, including various peripheral vascular diseases with tortuous anatomy," said Jean Bismuth, M.D., vascular surgeon at the Methodist DeBakey Heart and Vascular Center at The Methodist Hospital. "Additionally, the Magellan Robotic System may offer physicians less radiation exposure and reduced procedural fatigue due to the remote workstation that allows the physician to be seated comfortably outside the imaging suite.”
The company believes the Magellan platform also provides a compelling value proposition to hospitals that want to boost their profile with the regions they serve. Similar to the da Vinci surgical robotic system that many hospitals use as an advertising tool to draw more patients, the Magellan may have a similar marketing utility.
“Hospitals today need to ensure not only quality patient care, but also sound fiduciary judgment in all purchase decisions,” said Barclay. “To remain competitive in the markets they serve, hospitals need to become more efficient, while also increasing their patient capacity. The Magellan Robotic System has the potential to help hospitals accomplish both of these goals.”
The Magellan system received a CE Mark in the European Union last year. In addition, the system has been approved in Australia and is pending approval in Canada.
Hansen previously developed and released the Sensei robotic system to support long electrophysiology mapping and ablation procedures.
For more information: www.hansenmedical.com


 January 27, 2026
January 27, 2026