June 4, 2012 — MitrAssist Medical Ltd., a developer of minimally invasive products for treating heart disease, announced today it received ISO 13485:2003 certification for the design and development of implantable heart valve prostheses.
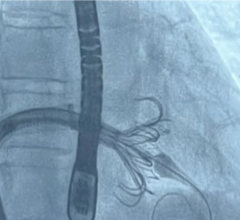
Today's standard treatment for mitral valve regurgitation (MR), blood leakage or backflow into the atrium, is open-heart surgery. MitrAssist's design preserves the ventricular dynamic mode of function and mechanism, known to be crucial for long-term patient outcome. MitrAssist's mitral valve prosthesis is delivered with a minimally invasive procedure via a small-diameter catheter, keeping the existing valve intact. With its design focused on natural mitral valve anatomy, the MitrAssist mitral valve prosthesis will enable faster transition to minimally invasive procedures for mitral repair.
"This is yet another small step for us moving forward towards developing a viable and effective solution to mitral valve insufficiency, following our successful completion of the chronic animal study POC," said Gil Naor, MitrAssist founder and CEO.
MitrAssist was founded in 2009 by Naor. It is a portfolio company of the Trendlines Group's Misgav Venture Accelerator. MitrAssist’s first product is a percutaneous mitral valve prosthesis for treatment of MR.
For more information: www.mitrassist.com


 September 04, 2024
September 04, 2024