December 28, 2011 – Hansen Medical, a developer of robotic technology for accurate 3-D control of catheter movement, announced the world's first uses of the Magellan robotic system in the treatment of peripheral vascular disease (PVD). The clinical procedures were performed by Professor Nick Cheshire, M.D., and the medical team at St. Mary's Hospital, part of the Imperial College Healthcare in London, pioneers in the use of flexible robotics in vascular interventions.
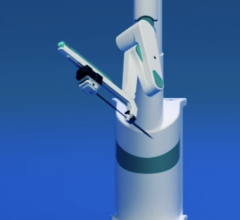
The Magellan robotic system is a new robotic approach in the treatment of vascular disease, and it has the potential to transform the way endovascular treatments are performed for patients with PVD. The system allows precise catheter navigation of peripheral vessels with a proprietary technology that enables independent distal tip control of a catheter and a sheath, as well as robotic manipulation of a standard guidewire from a centralized, remote workstation. The system was designed to allow precise and predictable catheter navigation of peripheral vessels while reducing procedure time, lessening radiation exposure, lowering procedural fatigue and enabling new procedures.
"Hansen Medical's new Magellan robotic system opens up the possibility for physicians to offer less invasive endovascular options to a broader group of patients suffering from complex disease," said Professor Nick Cheshire, head of circulation sciences at Imperial College Healthcare. "Imperial College has been pioneering the use of flexible robotics in vascular interventions, and the Magellan robotic system now gives us access to the latest robotic catheter technology that is specifically designed for peripheral vascular interventions."
Hansen Medical's Magellan robotic system is based upon the leading flexible robotic technology incorporated in the Sensei-X robotic catheter system currently sold in the U.S. and Europe, which has been used in nearly 7,000 patients with cardiac arrhythmia, but includes a number of key enhancements. In particular, the Magellan Robotic System:
- Allows for complete, individual robotic control of the distal tips of both the outer sheath and the inner leader catheter, as well as robotic manipulation of standard guidewires.
- Is designed to allow for sufficient extension inside the body to better access hard to reach peripheral anatomy.
- Preserves the open architecture featured in the Sensei system to allow for the subsequent use with most 6F therapeutic devices on the market today.
- Employs a catheter that is expected to be available in multiple lengths and has a low profile with significant flexibility to be compatible with most 6F treatment catheters currently used today.
Last year, Hansen announced the completion of its first-in-man study in Europe during which 20 endovascular procedures were successfully performed with an earlier version of the Magellan robotic system, demonstrating its potential to allow physicians to effectively treat peripheral vascular disease, while lessening radiation exposure.
In Europe, the Magellan robotic system and NorthStar robotic catheter are CE marked. In the United States, the Magellan robotic system requires U.S. Food & Drug Administration (FDA) clearance, and a 510(k) application is currently pending.
For more information: www.hansenmedical.com


 January 27, 2026
January 27, 2026