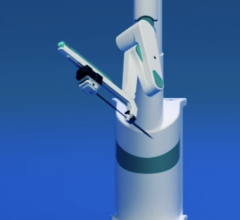
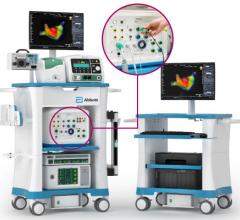
November 21, 2011 — Boston Scientific has reached an exclusive agreement with Catheter Robotics Inc. (CRI) to market the CRI Amigo system, a remote-controlled catheter system and related accessories. The two companies will focus their efforts in select European countries.
The CRI Amigo system is compatible with certain Boston Scientific and other commercially available catheters. It lets physicians remotely maneuver diagnostic and ablation catheters designed to treat common cardiac arrhythmias during electrophysiology procedures. Boston Scientific plans to market the Amigo system in Austria, Belgium, France, Germany, Luxembourg, The Netherlands, Portugal, Spain and Switzerland.
"To perform successful ablation procedures, electrophysiologists require precise control over catheter tip placement," said Jean Paul Albenque, M.D., Clinique Pasteur, Toulouse, France. "The Amigo Remote Catheter System, used in combination with the predictable steering and stability of Boston Scientific's leading RF ablation catheters, offers improved comfort and reduced radiation exposure without sacrificing performance."
The system is designed to integrate easily into existing cath labs for use in simple and complex procedures to diagnose and treat cardiac arrhythmias. It connects to the handle of specific electrophysiological catheters and lets the physician remotely operate the catheter with a controller. The system is intended to allow full operation of all catheter functions while reducing a physician's radiation exposure during the procedure.
The Amigo system is compatible with Boston Scientific's Blazer and Blazer Open-Irrigated radiofrequency (RF) ablation catheters and Chilli II fluid-cooled RF ablation catheter. The Blazer Open-Irrigated Catheter, launched in CE Mark countries in May 2011, is an advanced catheter that integrates Total Tip Cooling Design, which is intended to consistently cool the entire tip electrode during RF energy delivery.
Affecting more than 4.5 million Europeans, atrial fibrillation is an arrhythmia associated with a rapid rhythm in the upper chambers of the heart. Patients are most often treated with anti-arrhythmic drugs, which can often cause adverse side effects. Cardiac ablation with an RF ablation catheter is increasingly becoming an option for patients who cannot tolerate these medications.
In the United States, the Blazer Open-Irrigated Catheter is an investigational device, limited by applicable law to investigational use only and not available for sale.
For more information: www.bostonscientific.com, www.catheterrobotics.com


 January 27, 2026
January 27, 2026