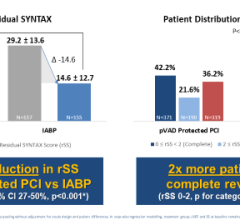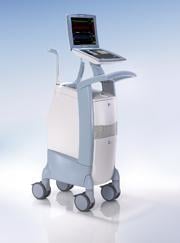
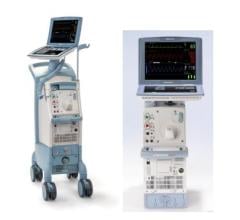
November 11, 2011 — Maquet Cardiovascular announced U.S. Food and Drug Administration (FDA) 510(k) clearance and CE mark for its new Cardiosave intra-aortic balloon pump (IABP). The announcement came at the 2011 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting in San Francisco. The system is expected to be commercially available in the United States in January 2012.
The new IABP incorporates a large state-of-the-art touchscreen display and is smaller, lighter and quieter than any pump the company has ever offered. The pump is being offered in two configurations: Cardiosave Hybrid for routine in-hospital use and Cardiosave Rescue for use in ambulances and aircraft.
Intra-aortic balloon counterpulsation is an adjunctive therapy often used in patients with left ventricular failure and other cardiac conditions. When the balloon is inserted into the patient's aorta, it counterpulsates with the heart and augments coronary blood flow to increase myocardial oxygen supply and decrease myocardial oxygen demand.
"The advanced user interface and improved patient interconnects make it very intuitive and easy to use," said Raoul Quintero, President, Maquet U.S. Sales and Service Organization. "Additionally, the ability to "hot swap" batteries allows virtually unlimited battery run time capability and allows patients who are being supported by the pump in one facility to be easily transported to another without any interruption in support regardless of the length of transport."
For more information: http://ca.maquet.com

 August 14, 2023
August 14, 2023