April 7, 2009 - In the European experience, the DuraHeart Left Ventricular Assist System (LVAS) showed sustained benefits in providing safe and reliable long-term circulatory support with an improved survival rate and an acceptable adverse event rate in advanced heart failure patients who were eligible for transplantation.
The conclusions are published online in the European Journal of Cardio-thoracic Surgery.
The European experience includes 68 patients who received the DuraHeart Left Ventricular Assist System between January 2004 and July 2008 including 33 patients treated as part of the European multi-center clinical trial (Germany, Austria, and France) and 35 patients treated in the post-trial period. All patients were classified with end-stage heart failure and were on approved lists to receive donor hearts (bridge to transplant). The trial sponsor was Terumo Heart Inc., maker of the DuraHeart System.
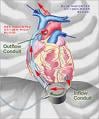
The DuraHeart System is a rotary blood pump designed for long-term patient support that incorporates a centrifugal flow rotary pump with an active magnetically levitated impeller featuring three position sensors and magnetic coils that reportedly optimize blood flow, while minimizing device wear and tear.
"In a patient population with advanced disease at imminent risk of death due to end-stage left ventricular failure and eligible for heart transplantation, the DuraHeart was safe and performed as intended," said Michiel Morshuis, M.D. senior physician, Clinic for Thoracic and Cardiovascular Surgery at the Heart and Diabetes Center North Rhine-Westphalia in Bad Oeynhausen. "We believe the device may have potential for long-term circulatory support not only as a bridge to transplant, but also for destination therapy."
As of the publication date, 35 patients (51 percent) in the study population remained in ongoing treatment with DuraHeart, 18 patients were transplanted, one device was explanted and 14 patients died during support with a median time to death of 62 days. The Kaplan-Meier survival rate during support for the 68 patients in the clinical trial and post-trial period was 81 percent at six months and 77 percent at one year.
DuraHeart LVAS is an investigational device and limited by U.S. law to investigational use.
For more information: www.terumoheart.com.


 February 06, 2026
February 06, 2026 









