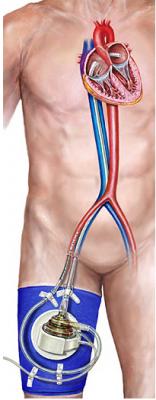
November 3, 2009 — CardiacAssist Inc. said yesterday the Cardiovascular Institute of Stanford University Hospital and Clinics in Palo Alto, Calif., is one of the newest cardiac centers to begin using TandemHeart extracorporeal circulatory support system.
TandemHeart is now being used in nearly 90 percent of the “Best U.S. Heart & Heart Surgery Hospitals” as ranked by U.S. News & World Report, with nearly 1,800 procedures performed at nearly 150 hospitals across the United States. Last month, CardiacAssist announced record nine-month financial results for 2009 compared to results for the same period in 2008.
TandemHeart can be placed rapidly by both interventional cardiologists in a cath lab and by cardiac surgeons in an operating room to provide short-term circulatory support to patients requiring additional cardiac assistance. The device provides effective and reliable temporary circulatory support for critically ill patients. Hemodynamic support includes a high net blood-flow rate of up to 5 liters per minute in the catheterization lab or up to 8 liters per minute in the OR and is fully reimbursed by Medicare under existing DRG codes.
Abiomed and Thoratec, TandemHeart has been added to the cardiac care programs at more than 20 centers in recent months.
Of the nearly 1,800 cases performed with TandemHeart to date by heart surgeons and cardiologists, 33 percent have been for ventricular dysfunction; 28 percent have been for high-risk cardiac surgery; and 39 percent have been for percutaneous cardiac interventions, the company said.
For more information: www.cardiacassist.com


 June 19, 2024
June 19, 2024 









