August 13, 2010 – A rotary blood pump designed for long-term patient support had another patient surpass four years after receiving the left ventricular assist device (LVAD). Helga Gieseke, 66, who lives in the south of Saxony-Anhalt, Germany is now one of the longest-living heart failure recipients of Terumo Heart’s DuraHeart Left Ventricular Assist System (LVAS).
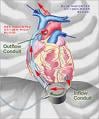
The DuraHeart LVAS is the latest generation rotary blood pump designed for long-term patient support. The system incorporates a centrifugal flow rotary pump with the magnetically levitated impeller. The pump features three position sensors and electromagnetic coils that suspend the impeller inside the pump chamber without a single contact point.
The impeller’s active magnetic levitation is designed to eliminate friction by allowing a wide gap between blood contacting surfaces, enabling blood to flow through the pump unimpeded in a smooth, non-turbulent fashion. The DuraHeart LVAS is currently being studied in the DuraHeart Pivotal U.S. Trial for Bridge-to-Transplant (BTT), a multicenter, prospective, nonrandomized study, involving 140 patients.
The system incorporates a centrifugal flow rotary pump with the magnetically levitated impeller. The pump features three position sensors and electromagnetic coils that suspend the impeller inside the pump chamber without a single contact point.
“When Mrs. Gieseke was admitted in our institution, she was in an advanced stage of chronic heart failure. Only an immediate heart transplant would have solved the problem, but a suitable donor heart was not available,” said professor Roland Hetzer, director of The Deutsches Herzzentrum Berlin (DHZB, German Heart Institute Berlin). “Instead, we explained that she would need a mechanical heart assist device and proposed that she participate in the DuraHeart LVAS clinical trial. She has done extremely well living with the DuraHeart LVAS, which has proven to be quite durable and capable of providing the critical long-term support required by our heart failure patients.”
The DuraHeart LVAS can be used as a bridge to heart transplant in patients with end stage heart failure. Due to the scarcity of donor organs, patients can sometimes be on the waiting list for many months until a suitable donor becomes available. During that time, a patient’s condition can deteriorate dramatically until no other alternative is available to them. The left ventricular assist systems offer the patient a second chance while waiting for a suitable donor
The impeller’s active magnetic levitation is designed to eliminate friction by allowing a wide gap between blood contacting surfaces, enabling blood to flow through the pump unimpeded in a smooth, nonturbulent fashion.
The device carries a CE mark and is currently available for sale in European countries. The company has completed clinical trial enrollment for this device in Japan. Additionally, Terumo Heart has submitted its destination therapy investigational device exemption to the U.S. Food and Drug Administration (FDA) to obtain approval to begin its DuraHeart DT Clinical Trial in the United States.
For more information: www.clinicaltrials.gov, www.terumoheart.com


 June 19, 2024
June 19, 2024 









