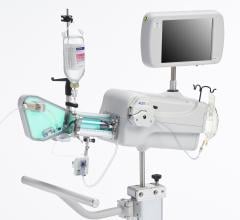
April 21, 2011 – A new intravascular ultrasound (IVUS) coronary imaging system has received the CE Mark. With the approval, the LipiScan IVUS system, from InfraReDx, is available for the detection of the plaques known to complicate stenting and believed to be the reason for most heart attacks.
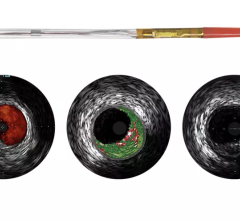
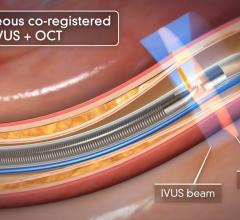
The system received U.S. Food and Drug Administration (FDA) approval in June 2010. It includes the world’s first and only cardiac catheter to combine intravascular ultrasound (IVUS) and near-infrared (NIR) spectroscopy to help cardiologists identify and characterize lipid core coronary plaques. In a single catheter pullback, it provides physicians with a traditional IVUS image that clearly displays key structural parameters of the lesion, including its location, length and degree of stenosis, in addition to confirming proper stent placement. At the same time, the system performs spectroscopic analysis of optical data to produce a Chemogram map that indicates the location of lipid core plaques and quantifies their lipid core burden. Integrating and co-registering the Chemogram with IVUS provides critical information to interventional cardiologists during the cardiac catheterization procedure.
“The LipiScan IVUS system is an important new intravascular imaging tool that will be of immediate value to interventional cardiologists for the diagnosis and management of their patients,” said Patrick W. Serruys, M.D., Ph.D., professor of interventional cardiology at the Thoraxcenter, Erasmus University Hospital, Rotterdam, Netherlands. “This impressive multimodality system provides an IVUS image of the vessel while simultaneously performing spectroscopic analysis for the accurate and immediate detection of lipid core plaques demonstrated to complicate stenting and suspected to cause most heart attacks. The Thoraxcenter is proud of its association with InfraReDx and the role we have played in the development of the LipiScan IVUS system and imaging catheter.”
For more information: www.infraredx.com
News | April 21, 2011


 September 18, 2025
September 18, 2025