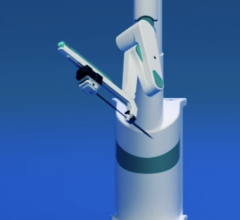
March 18, 2011 – A clinical trial testing a robotic system to remotely deliver and manipulating coronary guide wires has begun. The PRECISE study is testing the CorPath 200 System, from Corindus Vascular Robotics.
The first percutaneous coronary intervention (PCI) procedures were performed at New York Presbyterian Hospital/Columbia University Medical Center in New York and St. Elizabeth’s Medical Center in Boston.
“For the very first time in my career I was able to perform a PCI procedure without wearing a lead apron,” said Jeffrey W. Moses, M.D., investigator of the CorPath PRECISE trial, director of interventional cardiology at New York Presbyterian Hospital, director of the Center for Interventional Vascular Therapy at New York Presbyterian Hospital/Columbia University Medical Center. “The CorPath System’s radiation shielded cockpit provided an optimal view of the angiography screen and allowed me to easily manipulate the guide wire and accurately place the stent. I was impressed — this trial has the potential to significantly impact how we care for patients in the cath lab.”
The trial is a prospective, single-arm, multi-center study, which will initially enroll 154 patients. Investigators will evaluate the safety and effectiveness of the system in delivering and manipulating coronary guidewires and stent/balloon systems in PCI procedures. The trial is led by the co-principal investigators Giora Weisz, M.D., co-director of clinical services at the Center for Interventional Vascular Therapy at New York Presbyterian Hospital/Columbia University Medical Center and assistant professor of medicine at Columbia University College of Physicians and Surgeons, New York, and Joseph P. Carrozza, M.D., chief of cardiovascular medicine at St. Elizabeth’s Medical Center, Boston.
“I am pleased to lead and take part in a trial that is addressing a long time need in the cath lab — improved safety and ergonomics,” Carrozza said. “The comfortable seated position allowed me to focus solely on the patient’s physiology, while I was able to precisely control the guidewire and stent movements, even in 1 mm increments. Our first case was successful, and I look forward to performing more procedures using CorPath.”
The results of this study will be the basis for a 510(k) application to the U.S. Food and Drug Administration (FDA).
For more information: www.corindus.com


 January 27, 2026
January 27, 2026