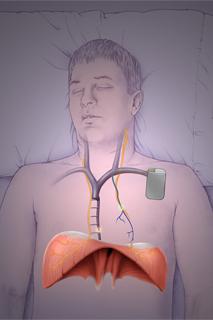
The RespiCardia System is implanted similar to a pacemaker.
January 24, 2011 – The first U.S. clinical implant of a device designed to restore more natural breathing patterns in patients with central sleep apnea was made last week at The Ohio State University Medical Center, Columbus. The procedure was performed by Ralph Augostini, M.D., assistant professor of clinical, cardiovascular medicine, on a 61-year-old male patient with a history of central sleep apnea and atrial fibrillation.
This was the first U.S. implant in a global pilot study of this novel therapy for treating a large and growing health problem. "The first U.S. implant of the RespiCardia System brings concept to reality," said William T. Abraham, M.D., professor of internal medicine and director of cardiovascular medicine at The Ohio State University. "The potential of this therapy is substantial, considering the very high prevalence of central sleep apnea in heart failure patients and in those with various neurological disorders."
It is estimated that about 35-40 percent of all heart failure patients have central sleep apnea. With the incidence of heart failure on the rise, there is even greater emphasis on diagnosing and treating this serious clinical problem.
"The technology has the potential to not only provide a valid alternative to positive pressure devices for the first time, but also to revolutionize the way we diagnose and treat sleep disorders in cardiac patients, " said Rami Khayat, M.D., associate professor of clinical, pulmonary/critical care at The Ohio State University.
The device is made by Cardiac Concepts Inc., which received CE mark approval in August 2010 for its use in Europe. The device is currently in U.S. clinical trials.
For more information visit www.cardiacconcepts.com


 January 15, 2026
January 15, 2026