October 18, 2010 – Toronto General Hospital is the first Canadian medical center to implant a left-ventricular assist system (LVAS) as part of a study for patients awaiting a heart transplant. The Peter Munk Cardiac Centre used the DuraHeart LVAS, by Terumo Heart, as part of the SUSTAIN BTT study.
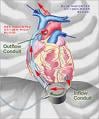
The DuraHeart LVAS is designed to aid the pumping action of the heart in order to circulate blood throughout the body, and is for use in patients with end-stage heart failure. Due to the scarcity of donor organs, patients can be on the waiting list for months until a suitable donor becomes available.
“We are pleased to be the first center in Canada to bring this state-of-the-art technology to our heart failure patients,” said Vivek Rao, M.D., surgical director of the heart transplant program at The Peter Munk Cardiac Centre, and DuraHeart LVAS clinical investigator. “We look forward to being part of the landmark SUSTAIN trial evaluating the DuraHeart LVAS, which we believe is one of the most advanced left-ventricular assist devices currently available.”
The system incorporates a centrifugal flow rotary pump with a magnetically levitated impeller. The pump features three position sensors and electromagnetic coils that suspend the impeller inside the pump chamber without a single point of contact. The impeller’s active magnetic levitation is designed to eliminate friction by allowing a wide gap between blood contacting surfaces, enabling blood to flow through the pump unimpeded in a smooth, non-turbulent fashion.
The study was granted unconditional approval in early 2010 by the U.S. Food & Drug Administration (FDA). The DuraHeart LVAS already carries a CE Mark and is currently available for sale in European countries. The company has recently completed clinical trial enrollment for this device in Japan, and has submitted its Destination Therapy (DT) Investigational Device Exemption to the FDA, which is a first step in the process of obtaining approval to begin its DuraHeart DT Clinical Trial in the United States.
For more information: www.terumoheart.com


 June 19, 2024
June 19, 2024 









