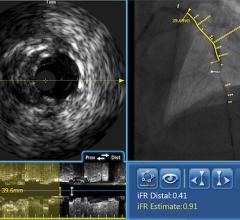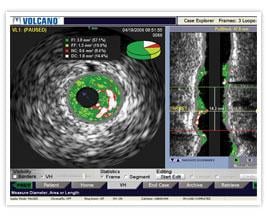
March 1, 2010 – The FDA recently granted 510(k) market clearance to the Volcano Corp. Eagle Eye Platinum digital intravascular ultrasound (IVUS) catheter.
The catheter offers the same functionality of its predecessor Eagle Eye Gold catheter, but offers improved deliverability and the convenience of additional radiopaque markers. Radiopaque markers were added to the catheter as a quick and easy way for physicians to estimate lengths without the need for a separate pullback device. Release of Eagle Eye Platinum in the U.S. is expected in the second quarter of 2010.
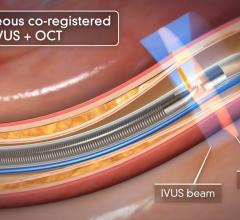
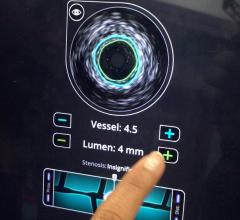
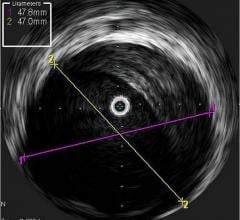
The Eagle Eye Platinum is the third generation of catheters in the Eagle Eye product line. To date, Eagle Eye catheters have been used in more than 500,000 procedures worldwide. The Eagle Eye Platinum catheter offers fast, plug-and-play functionality as well as grayscale IVUS, Virtual Histology (VH-IVUS), and ChromaFlo imaging modalities.
Clinical data continues to illustrate that angiography alone is often not enough to properly assess and treat coronary lesions. The STLLR (The Impact of Stent Deployment Techniques on Clinical Outcomes on Patients Treated with the Cypher Stent) trial was a double-blinded clinical study that showed improperly covering the length of a lesion or vessel blockage, is a contributing factor to poor stenting outcomes, stent thrombosis, and an increased risk of clinical events. In STLLR, where interventions were guided by angiography alone, optimal placement of the stent was achieved in only about half of the procedures.
For more information: www.volcanocorp.com


 September 18, 2025
September 18, 2025