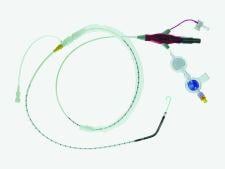
March 26, 2009 – Abiomed Inc. will exhibit its portfolio of heart recovery products spanning the continuum of cardiovascular patient care from the cath lab to the surgery suite at ACC 2009 in Orlando, FL, next week.
The Abiomed ACC booth will include daily demonstrations and hands-on simulation of its extensive heart recovery product suite, including: Impella 2.5, Impella 5.0, AB5000 portable driver and AB5000 Circulatory Support System.
The Impella 2.5 will be featured in five live cases at the ACC conference, including Columbia University Medical Center, Cedars-Sinai Medical Center, Saint Luke’s Hospital and Mid-America Heart Institute, and St. Vincent Hospital and the Heart Center of Indiana. Impella 2.5 will also be featured in a presentation made by physicians from Washington Hospital Center in Washington, DC.
For more information: www.abiomed.com


 June 19, 2024
June 19, 2024 









